- Record: found
- Abstract: found
- Article: found
Precision targeting of autoantigen-specific B cells in muscle-specific tyrosine kinase myasthenia gravis with chimeric autoantibody receptor T cells
Read this article at
Abstract
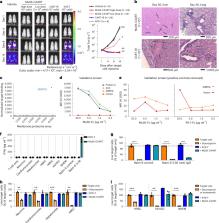
Muscle-specific tyrosine kinase myasthenia gravis (MuSK MG) is an autoimmune disease that causes life-threatening muscle weakness due to anti-MuSK autoantibodies that disrupt neuromuscular junction signaling. To avoid chronic immunosuppression from current therapies, we engineered T cells to express a MuSK chimeric autoantibody receptor with CD137-CD3ζ signaling domains (MuSK-CAART) for precision targeting of B cells expressing anti-MuSK autoantibodies. MuSK-CAART demonstrated similar efficacy as anti-CD19 chimeric antigen receptor T cells for depletion of anti-MuSK B cells and retained cytolytic activity in the presence of soluble anti-MuSK antibodies. In an experimental autoimmune MG mouse model, MuSK-CAART reduced anti-MuSK IgG without decreasing B cells or total IgG levels, reflecting MuSK-specific B cell depletion. Specific off-target interactions of MuSK-CAART were not identified in vivo, in primary human cell screens or by high-throughput human membrane proteome array. These data contributed to an investigational new drug application and phase 1 clinical study design for MuSK-CAART for the treatment of MuSK autoantibody-positive MG.
Abstract
A muscle autoimmune disease is treated in mice with CAAR T cells.
Related collections
Most cited references37
- Record: found
- Abstract: found
- Article: not found
Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia
- Record: found
- Abstract: found
- Article: not found
Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma
- Record: found
- Abstract: found
- Article: not found