- Record: found
- Abstract: found
- Article: not found
NAG-1/GDF15 prevents obesity by increasing thermogenesis, lipolysis and oxidative metabolism
Read this article at
Summary
Objective
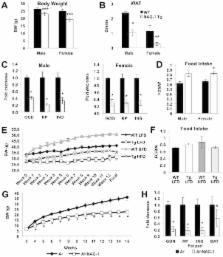
Obesity is a major health problem associated with high morbidity and mortality. NSAID activated gene, (NAG-1) is a TGF-β superfamily member reported to alter adipose tissue levels in mice. We investigated whether hNAG-1 acts as a regulator of adiposity and energy metabolism.
Design/Subjects
hNAG-1 mice, ubiquitously expressing hNAG-1, were placed on a control or high fat diet (HFD) for 12 weeks. hNAG-1 expressing B16/F10 melanoma cells were used in a xenograft model to deliver hNAG-1 to obese C57BL/6 mice.
Results
As compared to wild-type littermates, transgenic hNAG-1 mice have less white fat and brown fat despite equivalent food intake, improved glucose tolerance, lower insulin levels and are resistant to dietary- and genetic-induced obesity. hNAG-1 mice are more metabolically active with higher energy expenditure. Obese C57BL/6 mice treated with hNAG-1 expressing xenografts show decreases in adipose tissue and serum insulin levels. hNAG-1 mice and obese mice treated with hNAG-1 expressing xenografts show increased thermogenic gene expression ( UCP1, PGC1α, ECH1, Cox8b, Dio2, Cyc1, PGC1β, PPARα, Elvol3) in brown adipose tissue (BAT) and increased expression of lipolytic genes ( Adrb3, ATGL, HSL) in both white adipose tissue (WAT) and BAT, consistent with higher energy metabolism
Related collections
Most cited references38
- Record: found
- Abstract: found
- Article: not found
Adipose tissue dysfunction in obesity, diabetes, and vascular diseases.
- Record: found
- Abstract: found
- Article: not found
Brown adipose tissue oxidative metabolism contributes to energy expenditure during acute cold exposure in humans.
- Record: found
- Abstract: found
- Article: not found
