- Record: found
- Abstract: found
- Article: found
Radiation-induced changes to bone composition extend beyond periosteal bone
Read this article at
Abstract
Background
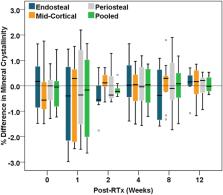
Cancer patients receiving radiotherapy for soft tissue sarcomas are often at risk of post-irradiation (post-RTx) bone fragility fractures, but our understanding of factors controlling radiation-induced bone injury is limited. Previous studies have evaluated post-RTx changes to cortical bone composition in the periosteum of irradiated tibiae, but have not evaluated effects of irradiation in deeper tissues, such as endosteal or mid-cortical bone, and whether there are differential spatial effects of irradiation. In this study, we hypothesize that post-RTx changes to cortical bone composition are greater in endosteal compared to mid-cortical or periosteal bone.
Methods
A pre-clinical mouse model of limited field hindlimb irradiation was used to evaluate spatial and temporal post-RTx changes to the metaphyseal cortex of irradiated tibiae. Irradiation was delivered unilaterally to the hindlimbs of 12-wk old female BALB/cJ mice as 4 consecutive daily doses of 5 Gy each. RTx and non-RTx tibiae were obtained at 0, 2, 4, 8, and 12 wks post-RTx ( n = 9 mice/group/time). Raman spectroscopy was used to evaluate spatial and temporal post-RTx changes to cortical bone composition in age-matched RTx and non-RTx groups.
Results
Significant early spatial differences in mineral/matrix and collagen crosslink ratios were found between endosteal and periosteal or mid-cortical bone at 2-wks post-RTx. Although spatial differences were transient, mineral/matrix ratios significantly decreased and collagen crosslink ratios significantly increased with post-RTx time throughout the entire tibial metaphyseal cortex.
Conclusions
Irradiation negatively impacts the composition of cortical bone in a spatially-dependent manner starting as early as 2-wks post-RTx. Long-term progressive post-RTx changes across all cortical bone sites may eventually contribute to the increased risk of post-RTx bone fragility fractures.
Related collections
Most cited references61
- Record: found
- Abstract: found
- Article: not found
Spectroscopic characterization of collagen cross-links in bone.
- Record: found
- Abstract: found
- Article: not found
Carbonate assignment and calibration in the Raman spectrum of apatite.
- Record: found
- Abstract: found
- Article: not found