- Record: found
- Abstract: found
- Article: found
Lomerizine inhibits LPS-mediated neuroinflammation and tau hyperphosphorylation by modulating NLRP3, DYRK1A, and GSK3α/β

Read this article at
Abstract
Introduction
Lomerizine is a calcium channel blocker that crosses the blood–brain barrier and is used clinically in the treatment of migraines. However, whether lomerizine is beneficial in modulating neuroinflammatory responses has not been tested yet.
Methods
To assess the potential of lomerizine for repurposing as a treatment for neuroinflammation, we investigated the effects of lomerizine on LPS-induced proinflammatory responses in BV2 microglial cells, Alzheimer’s disease (AD) excitatory neurons differentiated from induced pluripotent stem cells (iPSCs), and in LPS-treated wild type mice.
Results
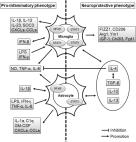
In BV2 microglial cells, lomerizine pretreatment significantly reduced LPS-evoked proinflammatory cytokine and NLRP3 mRNA levels. Similarly, lomerizine pretreatment significantly suppressed the increases in Iba-1, GFAP, proinflammatory cytokine and NLRP3 expression induced by LPS in wild-type mice. In addition, lomerizine posttreatment significantly decreased LPS-stimulated proinflammatory cytokine and SOD2 mRNA levels in BV2 microglial cells and/or wild-type mice. In LPS-treated wild-type mice and AD excitatory neurons differentiated from iPSCs, lomerizine pretreatment ameliorated tau hyperphosphorylation. Finally, lomerizine abolished the LPS-mediated activation of GSK3α/β and upregulation of DYRK1A, which is responsible for tau hyperphosphorylation, in wild-type mice.
Related collections
Most cited references62
- Record: found
- Abstract: not found
- Article: not found
Cytokines, inflammation, and pain.
- Record: found
- Abstract: found
- Article: found
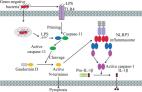
Recent advances in the mechanisms of NLRP3 inflammasome activation and its inhibitors
- Record: found
- Abstract: found
- Article: found