- Record: found
- Abstract: found
- Article: found
A Review of the Role of Bioreactors for iPSCs-Based Tissue-Engineered Articular Cartilage
Read this article at
Abstract
Background:
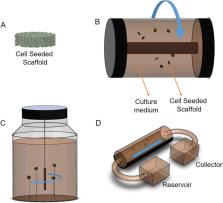
Osteoarthritis (OA) is the most common degenerative joint disease without an ultimate treatment. In a search for novel approaches, tissue engineering (TE) has shown great potential to be an effective way for hyaline cartilage regeneration and repair in advanced stages of OA. Recently, induced pluripotent stem cells (iPSCs) have been appointed to be essential stem cells for degenerative disease treatment because they allow a personalized medicine approach. For clinical translation, bioreactors in combination with iPSCs-engineerd cartilage could match patients needs, serve as platform for large-scale patient specific cartilage production, and be a tool for patient OA modelling and drug screening. Furthermore, to minimize in vivo experiments and improve cell differentiation and cartilage extracellular matrix (ECM) deposition, TE combines existing approaches with bioreactors.
Methods:
This review summarizes the current understanding of bioreactors and the necessary parameters when they are intended for cartilage TE, focusing on the potential use of iPSCs.
Results:
Bioreactors intended for cartilage TE must resemble the joint cavity niche. However, recreating human synovial joints is not trivial because the interactions between various stimuli are not entirely understood.
Conclusion:
The use of mechanical and electrical stimulation to differentiate iPSCs, and maintain and test chondrocytes are key stimuli influencing hyaline cartilage homeostasis. Incorporating these stimuli to bioreactors can positively impact cartilage TE approaches and their possibility for posterior translation into the clinics.
Related collections
Most cited references80
- Record: found
- Abstract: found
- Article: not found
Induction of pluripotent stem cells from adult human fibroblasts by defined factors.
- Record: found
- Abstract: found
- Article: not found
Capturing complex 3D tissue physiology in vitro.
