- Record: found
- Abstract: found
- Article: found
Validation of the questionnaire of olfactory disorders (QOD) for the Brazilian population
Highlights
Abstract
Background
The incidence of olfactory disorders has increased in recent years, mainly related to COVID-19 infection. In Brazil, over 37 million cases of COVID-19 have been reported, and approximately 10 % of those cases continue to experience olfactory disorders for more than one month. Despite the significant negative impact on well-being, there is currently no validated instrument to assess how olfactory disorders impact the quality of life in Brazil.
Objectives
This study aimed to validate the Questionnaire of Olfactory Disorders (QOD) for Brazilian Portuguese.
Methods
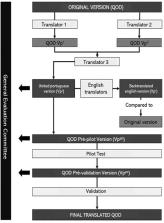
The authors first performed translation, back-translation, expert review, pre-testing, psychometric evaluation and cultural adaptation of the English version of the questionnaire. To assure linguistic and conceptual equivalence of the translated questionnaire, 126 participants from two Brazilian states and varying degrees of olfactory loss answered the QOD and the World Health Organization Quality of Life bref (WHOQOL-bref) questionnaires. The University of Pennsylvania Smell Identification Test (UPSIT®) was used to quantify the olfactory loss. Furthermore, to evaluate the reliability of the Portuguese version a test-retest was performed on a subgroup of patients. The authors observed a high Cronbach's alpha (α = 0.86) for internal consistency of the quality of Life (QOD-QOL) statements.
Findings
As expected, there was a negative correlation between QOD-QOL and UPSIT® (Spearman's ρ = -0.275, p = 0.002), since QOL score increases and UPSIT® score decreases with worsening of olfactory function. Correlations were moderate between QOD-QOL and WHOQOL-bref mean (Spearman's ρ = -0.374, p < 0.001) and weak to moderate between the QOD-QOL and Visual Analog Scale of the QOD regarding professional life, leisure, and private life (Spearman's ρ = -0.316, p = 0.000; Spearman's ρ = -0.293, p = 0.001; Spearman's ρ = -0.261, p = 0.004; respectively).
Related collections
Most cited references36
- Record: found
- Abstract: found
- Article: not found
6-month consequences of COVID-19 in patients discharged from hospital: a cohort study
- Record: found
- Abstract: found
- Article: not found
The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection.
- Record: found
- Abstract: not found
- Article: not found