- Record: found
- Abstract: found
- Article: found
Exosomes derived from human adipose mesenchymal stem cells improve ovary function of premature ovarian insufficiency by targeting SMAD

Read this article at
Abstract
Background
Although many reports show that various kinds of stem cells have the ability to recover the function of premature ovarian insufficiency (POI), few studies are associated with the mechanism of stem cell treatment of POI. We designed this experimental study to investigate whether human adipose stem cell-derived exosomes (hADSC-Exos) retain the ability to restore ovarian function and how hADSC-Exos work in this process.
Methods
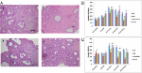
A POI mouse model was established and human ovarian granule cells (hGCs) collected from individuals with POI were prepared to assess the therapeutic effects and illuminate the mechanism of hADSCs in curing POI. The hematoxylin and eosin assay method was employed to assess the number of follicles. Enzyme-linked immunosorbent assay (ELISA) was used to detect the serum levels of sex hormones. The proliferation rate and marker expression levels of hGCs were measured by flow cytometry (fluorescence-activated cell sorting). Real-time PCR and western blot assays were used to determine the mRNA and protein expression levels of SMAD2, SMAD3, and SMAD5. Western blot assays were used to test the protein expression levels of apoptosis genes (Fas, FasL, caspase-3, and caspase-8).
Results
After the hADSC-Exos were transplanted into the POI mice model, they exerted better therapeutic activity on mouse ovarian function, improving follicle numbers during four stages. ELISA results showed that hADSC-Exos elevated the hormone levels to the normal levels. In addition, after hADSC-Exos were cocultured with POI hGCs, our results showed that hADSC-Exos significantly promoted the proliferation rate and inhibited the apoptosis rate. Furthermore, hADSC-Exos also increased the marker expression of hGCs to the normal level. Besides, mRNA and protein assays demonstrated that hADSC-Exos downregulated the expression of SMAD2, SMAD3, and SMAD5 in vivo and in vitro. Western blot assay demonstrated that hADSC-Exos inhibited expression of the apoptosis genes in POI hGCs, and SMAD knockdown increased the protein expression of apoptosis genes.
Related collections
Most cited references19

- Record: found
- Abstract: found
- Article: found
Exosomes derived from human menstrual blood-derived stem cells alleviate fulminant hepatic failure
- Record: found
- Abstract: found
- Article: found
Adipose-derived stem cells improved mouse ovary function after chemotherapy-induced ovary failure

- Record: found
- Abstract: found
- Article: found