- Record: found
- Abstract: found
- Article: found
Effects of Nt-truncation and coexpression of isolated Nt domains on the membrane trafficking of electroneutral Na +/HCO 3 – cotransporters
Read this article at
Abstract
The SLC4 genes are all capable of producing multiple variants by alternative splicing
or using alternative promoters. The physiological consequences of such diversity are
of great interest to investigators. Here, we identified two novel variants of the
electroneutral Na
+/
 cotransporter NBCn1, one full-length starting with “MIPL” and the other Nt-truncated
starting with “MDEL”. Moreover, we identified a new promoter of
Slc4a10 encoding NBCn2 and a novel type of Nt-truncated NBCn2 starting with “MHAN”. When
heterologously expressed, the new NBCn1 variants were well localized to the plasma
membrane and exhibited characteristic NBCn1 activity. However, MHAN-NBCn2 was poorly
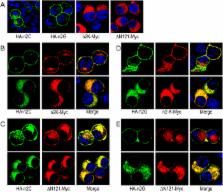
localized on the plasma membrane. By deletion mutations, we identified the Nt regions
important for the surface localization of NBCn2. Interestingly, coexpressing the full-length
NBCn2 greatly enhances the surface abundance of the Nt-truncated NBCn2. Co-immunoprecipitation
and bimolecular fluorescence complementation studies showed that the full-length and
Nt-truncated NBCn2 interact with each other to form heterodimers in neuro-2A cells.
Finally, we showed that the isolated Nt domain interacts with and enhances the surface
abundance of the Nt-truncated NBCn2. The present study expands our knowledge of the
NBCn1 and NBCn2 transcriptome, and provides insights into how the Nt domain could
affect transporter function by regulating its membrane trafficking.
cotransporter NBCn1, one full-length starting with “MIPL” and the other Nt-truncated
starting with “MDEL”. Moreover, we identified a new promoter of
Slc4a10 encoding NBCn2 and a novel type of Nt-truncated NBCn2 starting with “MHAN”. When
heterologously expressed, the new NBCn1 variants were well localized to the plasma
membrane and exhibited characteristic NBCn1 activity. However, MHAN-NBCn2 was poorly
localized on the plasma membrane. By deletion mutations, we identified the Nt regions
important for the surface localization of NBCn2. Interestingly, coexpressing the full-length
NBCn2 greatly enhances the surface abundance of the Nt-truncated NBCn2. Co-immunoprecipitation
and bimolecular fluorescence complementation studies showed that the full-length and
Nt-truncated NBCn2 interact with each other to form heterodimers in neuro-2A cells.
Finally, we showed that the isolated Nt domain interacts with and enhances the surface
abundance of the Nt-truncated NBCn2. The present study expands our knowledge of the
NBCn1 and NBCn2 transcriptome, and provides insights into how the Nt domain could
affect transporter function by regulating its membrane trafficking.
Related collections
Most cited references28
- Record: found
- Abstract: found
- Article: not found
Visualization of interactions among bZIP and Rel family proteins in living cells using bimolecular fluorescence complementation.
- Record: found
- Abstract: found
- Article: not found
The SLC4 family of bicarbonate (HCO₃⁻) transporters.
- Record: found
- Abstract: found
- Article: not found