- Record: found
- Abstract: found
- Article: found
Enhancing the Response Rate to Recombinant Uricases in Patients with Gout
Read this article at
Abstract
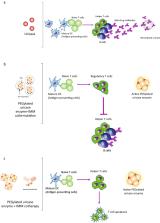
Refractory, or uncontrolled, gout is a chronic, progressive, inflammatory arthropathy resulting from continued urate deposition after failed attempts to lower serum uric acid below the therapeutic threshold with oral urate-lowering therapies such as allopurinol and febuxostat. Recombinant uricase is increasingly being used to treat refractory gout; however, the immunogenicity of uricase-based therapies has limited the use of these biologic therapies. Antidrug antibodies against biologic therapies, including uricase and PEGylated uricase, can lead to loss of urate-lowering response, increased risk of infusion reactions, and subsequent treatment failure. However, co-therapy with an immunomodulator can attenuate antidrug antibody development, potentially increasing the likelihood of sustained urate lowering, therapy course completion, and successful treatment outcomes. This review summarizes evidence surrounding the use of immunomodulation as co-therapy with recombinant uricases.
Related collections
Most cited references59

- Record: found
- Abstract: found
- Article: found
Update on the Pathomechanism, Diagnosis, and Treatment Options for Rheumatoid Arthritis
- Record: found
- Abstract: found
- Article: not found
2020 American College of Rheumatology Guideline for the Management of Gout
- Record: found
- Abstract: found
- Article: not found