- Record: found
- Abstract: found
- Article: found
APOE and Alzheimer’s Disease: Advances in Genetics, Pathophysiology, and Therapeutic Approaches.
Read this article at
SUMMARY
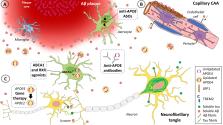
The APOE ε4 allele remains the strongest genetic risk factor for sporadic Alzheimer’s disease and the APOE ε2 allele the strongest genetic protective factor after multiple large scale genome-wide association studies and genome-wide association meta-analyses. However, no therapies directed at APOE are currently available. Although initial studies causally linked APOE with amyloid-β peptide aggregation and clearance, over the past 5 years our understanding of APOE pathogenesis has expanded beyond amyloid-β peptide-centric mechanisms to tau neurofibrillary degeneration, microglia and astrocyte responses, and blood-brain barrier disruption. Because all these pathological processes can potentially contribute to cognitive impairment, it is important to use this body of knowledge to develop therapies directed at APOE. Several therapeutic approaches have been successful in mouse models expressing human APOE alleles, including increasing or reducing APOE levels, enhancing its lipidation, blocking the interactions between APOE and amyloid-β peptide, and genetically switching APOE4 to APOE3 or APOE2 isoforms, but translation to human clinical trials has proven challenging.
Related collections
Most cited references100
- Record: found
- Abstract: found
- Article: not found
A Unique Microglia Type Associated with Restricting Development of Alzheimer's Disease.
- Record: found
- Abstract: found
- Article: found
Genetic meta-analysis of diagnosed Alzheimer’s disease identifies new risk loci and implicates Aβ, tau, immunity and lipid processing
- Record: found
- Abstract: found
- Article: not found
Single-cell transcriptomic analysis of Alzheimer’s disease
Author and article information
Comments
Comment on this article
 Smart Citations
Smart CitationsSee how this article has been cited at scite.ai
scite shows how a scientific paper has been cited by providing the context of the citation, a classification describing whether it supports, mentions, or contrasts the cited claim, and a label indicating in which section the citation was made.
