- Record: found
- Abstract: found
- Article: found
Dysregulation of mitochondria-lysosome contacts by GBA1 dysfunction in dopaminergic neuronal models of Parkinson’s disease
Read this article at
Abstract
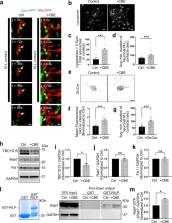
Mitochondria-lysosome contacts are recently identified sites for mediating crosstalk between both organelles, but their role in normal and diseased human neurons remains unknown. In this study, we demonstrate that mitochondria-lysosome contacts can dynamically form in the soma, axons, and dendrites of human neurons, allowing for their bidirectional crosstalk. Parkinson’s disease patient derived neurons harboring mutant GBA1 exhibited prolonged mitochondria-lysosome contacts due to defective modulation of the untethering protein TBC1D15, which mediates Rab7 GTP hydrolysis for contact untethering. This dysregulation was due to decreased GBA1 (β-glucocerebrosidase (GCase)) lysosomal enzyme activity in patient derived neurons, and could be rescued by increasing enzyme activity with a GCase modulator. These defects resulted in disrupted mitochondrial distribution and function, and could be further rescued by TBC1D15 in Parkinson’s patient derived GBA1-linked neurons. Together, our work demonstrates a potential role of mitochondria-lysosome contacts as an upstream regulator of mitochondrial function and dynamics in midbrain dopaminergic neurons in GBA1-linked Parkinson’s disease.
Abstract
Mitochondria-lysosome contact sites mediate cross-talk between the two organelles. Here, the authors show mitochondria-lysosome contacts are prolonged and defective in heterozygous mutant GBA1 neurons, which is caused by defective modulation of TBC1D15 due to decreased GBA1 activity.
Related collections
Most cited references65
- Record: found
- Abstract: found
- Article: not found
Genome engineering using the CRISPR-Cas9 system.
- Record: found
- Abstract: found
- Article: not found
The roles of PINK1, parkin, and mitochondrial fidelity in Parkinson's disease.
- Record: found
- Abstract: found
- Article: not found