- Record: found
- Abstract: found
- Article: found
Risk for In-Hospital Complications Associated with COVID-19 and Influenza — Veterans Health Administration, United States, October 1, 2018–May 31, 2020
research-article
Jordan Cates , PhD
1
,
2
,
,
Cynthia Lucero-Obusan , MD
3 ,
Rebecca M. Dahl , MPH
1 ,
Patricia Schirmer , MD
3 ,
Shikha Garg , MD
1
,
4 ,
Gina Oda , MS
3 ,
Aron J. Hall , DVM
1 ,
Gayle Langley , MD
1 ,
Fiona P. Havers , MD
1 ,
Mark Holodniy , MD
3
,
5 ,
Cristina V. Cardemil , MD
1
,
4
23 October 2020
Read this article at
There is no author summary for this article yet. Authors can add summaries to their articles on ScienceOpen to make them more accessible to a non-specialist audience.
Abstract
On October 20, 2020, this report was posted as an MMWR Early Release on the MMWR website
(https://www.cdc.gov/mmwr).
Coronavirus disease 2019 (COVID-19) is primarily a respiratory illness, although increasing
evidence indicates that infection with SARS-CoV-2, the virus that causes COVID-19,
can affect multiple organ systems (
1
). Data that examine all in-hospital complications of COVID-19 and that compare these
complications with those associated with other viral respiratory pathogens, such as
influenza, are lacking. To assess complications of COVID-19 and influenza, electronic
health records (EHRs) from 3,948 hospitalized patients with COVID-19 (March 1–May
31, 2020) and 5,453 hospitalized patients with influenza (October 1, 2018–February
1, 2020) from the national Veterans Health Administration (VHA), the largest integrated
health care system in the United States,* were analyzed. Using International Classification
of Diseases, Tenth Revision,
Clinical Modification (ICD-10-CM) codes, complications in patients with laboratory-confirmed
COVID-19 were compared with those in patients with influenza. Risk ratios were calculated
and adjusted for age, sex, race/ethnicity, and underlying medical conditions; proportions
of complications were stratified among patients with COVID-19 by race/ethnicity. Patients
with COVID-19 had almost 19 times the risk for acute respiratory distress syndrome
(ARDS) than did patients with influenza, (adjusted risk ratio [aRR] = 18.60; 95% confidence
interval [CI] = 12.40–28.00), and more than twice the risk for myocarditis (2.56;
1.17–5.59), deep vein thrombosis (2.81; 2.04–3.87), pulmonary embolism (2.10; 1.53–2.89),
intracranial hemorrhage (2.85; 1.35–6.03), acute hepatitis/liver failure (3.13; 1.92–5.10),
bacteremia (2.46; 1.91–3.18), and pressure ulcers (2.65; 2.14–3.27). The risks for
exacerbations of asthma (0.27; 0.16–0.44) and chronic obstructive pulmonary disease
(COPD) (0.37; 0.32–0.42) were lower among patients with COVID-19 than among those
with influenza. The percentage of COVID-19 patients who died while hospitalized (21.0%)
was more than five times that of influenza patients (3.8%), and the duration of hospitalization
was almost three times longer for COVID-19 patients. Among patients with COVID-19,
the risk for respiratory, neurologic, and renal complications, and sepsis was higher
among non-Hispanic Black or African American (Black) patients, patients of other races,
and Hispanic or Latino (Hispanic) patients compared with those in non-Hispanic White
(White) patients, even after adjusting for age and underlying medical conditions.
These findings highlight the higher risk for most complications associated with COVID-19
compared with influenza and might aid clinicians and researchers in recognizing, monitoring,
and managing the spectrum of COVID-19 manifestations. The higher risk for certain
complications among racial and ethnic minority patients provides further evidence
that certain racial and ethnic minority groups are disproportionally affected by COVID-19
and that this disparity is not solely accounted for by age and underlying medical
conditions.
The study population comprised two cohorts of hospitalized adult (aged ≥18 years)
VHA patients: 1) those with nasopharyngeal (90%) or other specimens that had tested
positive for SARS-CoV-2 by real-time reverse transcription–polymerase chain reaction
(RT-PCR) during March 1–May 31, 2020, and 2) those with laboratory-confirmed influenza
A or B by rapid antigen assay, real-time RT-PCR, direct or indirect fluorescent staining,
or viral culture, during October 1, 2018–February 1, 2020. Patients who received an
influenza diagnosis after February 1, 2020, were excluded to minimize the possible
inclusion of patients co-infected with SARS-CoV-2. Patients were restricted to those
with a COVID-19 or influenza test during hospitalization or in the 30 days preceding
hospitalization (including inpatient care at a nursing home). Patients who were still
hospitalized as of July 31, 2020, or who were admitted >14 days before receiving testing
were excluded from the analysis.
Data from EHRs were extracted from VHA Praedico Surveillance System, a biosurveillance
application used for early detection, monitoring, and forecasting of infectious disease
outbreaks
†
and Corporate Data Warehouse. Data included age, sex, race/ethnicity, ICD-10-CM diagnosis
codes, hospital admission and discharge date, and, if applicable, date of intensive
care unit (ICU) admission and date of death. Thirty-three acute complications (not
mutually exclusive) were identified using ICD-10-CM codes from the hospitalization
EHR (
2
). Underlying medical conditions were identified using ICD-10-CM codes from inpatient,
outpatient, and problem list records from at least 14 days before the specimen collection
date (
3
).
Categorical variables were compared using Chi-squared or Fisher’s exact test and continuous
variables with Wilcoxon rank sum test. Two-sided p-values <0.05 were considered statistically
significant. Among patients with COVID-19, the risk for complications was compared
among racial/ethnic groups using log-binomial models, adjusting for age and underlying
medical conditions, with White patients as the reference group. Relative risk for
complications in patients with COVID-19 compared with those with influenza were estimated
using log-binomial models, adjusting for age, sex, race/ethnicity, and underlying
medical conditions. To assess bias from seasonality in complications unrelated to
influenza or COVID-19, a sensitivity analysis restricted to cases diagnosed during
March–May of 2019 (influenza) and March–May of 2020 (COVID-19) was conducted. All
analyses were performed using SAS (version 9.4; SAS Institute). The data used in this
analysis were obtained for the purpose of public health operations in VHA.
§
Because no additional analyses were performed outside public health operational activities,
the activity was determined to meet the requirements of public health surveillance
as defined in 45 CFR 46.102(l)(2), and Institutional Review Board review was not required.
During October 1, 2018–February 1, 2020, 5,746 hospitalized patients received a positive
influenza test result and during March 1–May 31, 2020, 4,305 hospitalized patients
received a positive SARS-CoV-2 test result. For both groups, testing occurred during
the 30 days preceding hospitalization or while hospitalized. A total of 132 patients
admitted >14 days before testing were excluded, as were 518 patients who were still
hospitalized as of July 31, 2020, leaving 5,453 influenza patients and 3,948 COVID-19
patients for analysis.
Patients with COVID-19 were slightly older than were those with influenza (median = 70
years; interquartile range [IQR] = 61–77 years versus 69 years; IQR = 61–75 years)
(p = 0.001), but patients with influenza had higher prevalences of most underlying
medical conditions than did those with COVID-19 (Table 1). Black patients accounted
for 48.3% of COVID-19 patients and 24.7% of influenza patients; the proportion of
Hispanic patients was similar in both groups. The percentage of COVID-19 patients
admitted to an ICU (36.5%) was more than twice that of influenza patients (17.6%);
the percentage of COVID-19 patients who died while hospitalized (21.0%) was more than
five times that of influenza patients (3.8%); and the duration of hospitalization
was almost three times longer for COVID-19 patients (median 8.6 days; IQR = 3.9–18.6
days) than that for influenza patients (3.0 days; 1.8–6.5 days) (p<0.001 for all).
TABLE 1
Demographics, underlying medical conditions, acute complications, and hospital outcomes
among hospitalized patients with COVID-19 (March 1–May 31, 2020) and among historically
hospitalized patients with influenza (October 1, 2018–February 1, 2020)* — Veterans
Health Administration, United States
Characteristic or condition
No. (%)
P-value
COVID-19
Influenza
Baseline characteristics
No. of patients
3,948
5,453
—
Median age at test date, yrs (IQR)
70 (61–77)
69 (61–75)
0.001
Male
3,710 (94.0)
5,116 (93.8)
0.76
Race/Ethnicity
White, non-Hispanic
1,515 (40.4)
3,389 (64.0)
<0.001
Black, non-Hispanic
1,811 (48.3)
1,305 (24.7)
Other race, non-Hispanic†
87 (2.3)
150 (2.8)
Hispanic or Latino
336 (9.0)
449 (8.5)
Underlying medical conditions§
Asthma
260 (6.9)
565 (10.5)
<0.001
COPD
903 (23.9)
2,261 (42.0)
<0.001
Other lung conditions
534 (14.1)
1,078 (20.0)
<0.001
Blood disorders
123 (3.2)
257 (4.8)
<0.001
Cerebrovascular diseases
468 (12.4)
558 (10.4)
<0.001
Heart disease
1,909 (50.4)
3,068 (57.0)
<0.001
Heart failure
707 (18.7)
1,320 (24.5)
<0.001
Hypertension
2,893 (76.4)
4,082 (75.9)
0.77
Diabetes mellitus
1,873 (49.5)
2,416 (44.9)
<0.001
Renal conditions
1,111 (29.4)
1,468 (27.3)
0.03
Liver diseases
528 (13.9)
687 (12.8)
0.10
Immunosuppression
537 (14.2)
1,033 (19.2)
<0.001
Long-term medication use
451 (11.9)
776 (14.4)
<0.001
Cancer
696 (18.4)
1,341 (24.9)
<0.001
Neurologic/Musculoskeletal
1,602 (42.3)
2,091 (38.9)
<0.001
Endocrine disorders
620 (16.4)
996 (18.5)
0.01
Metabolic conditions
2,525 (66.7)
3,628 (67.5)
0.45
Extreme obesity
333 (8.8)
518 (9.6)
0.18
Any underlying medical condition¶
3,541 (93.6)
5,117 (95.1)
0.001
In-hospital complications**
Respiratory
3,030 (76.8)
5,167 (94.8)
<0.001
Pneumonia
2,766 (70.1)
1,916 (35.1)
<0.001
Respiratory failure
1,834 (46.5)
1,556 (28.5)
<0.001
ARDS
369 (9.3)
29 (0.5)
<0.001
Asthma exacerbation, no./No. (%)††
17/260 (6.5)
127/565 (22.5)
<0.001
COPD exacerbation, no./No. (%)††
160/903 (17.7)
1,154/2,261 (51.0)
<0.001
Pneumothorax
24 (0.6)
9 (0.2)
<0.001
Cardiovascular
516 (13.1)
911 (16.7)
<0.001
Acute MI/Unstable angina
300 (7.6)
499 (9.2)
0.01
Acute CHF
216 (5.5)
467 (8.6)
<0.001
Cardiogenic shock
36 (0.9)
28 (0.5)
0.02
Hypertensive crisis
53 (1.3)
90 (1.7)
0.23
Acute myocarditis
23 (0.6)
11 (0.2)
0.002
Hematologic
244 (6.2)
135 (2.5)
<0.001
Deep vein thrombosis
131 (3.3)
62 (1.1)
<0.001
Pulmonary embolism
112 (2.8)
72 (1.3)
<0.001
DIC
18 (0.5)
6 (0.1)
0.001
Neurologic
161 (4.1)
116 (2.1)
<0.001
Cerebral ischemia/infarction
125 (3.2)
92 (1.7)
<0.001
Intracranial hemorrhage
27 (0.7)
10 (0.2)
<0.001
Endocrine
79 (2.0)
80 (1.5)
0.05
Diabetic ketoacidosis, no./No. (%)††
42/1,873 (2.2)
42/2,416 (1.7)
0.24
Gastrointestinal
77 (2.0)
200 (3.7)
<0.001
Acute hepatitis/liver failure
63 (1.6)
26 (0.5)
<0.001
Renal
1,562 (39.6)
1,434 (26.3)
<0.001
Acute kidney failure
1,541 (39.0)
1,413 (25.9)
<0.001
Dialysis initiation§§
120 (3.0)
39 (0.7)
<0.001
Other¶¶
1,249 (31.6)
1,258 (23.1)
<0.001
Sepsis
984 (24.9)
1,012 (18.6)
<0.001
Bacteremia
186 (4.7)
100 (1.8)
<0.001
Pressure ulcer
289 (7.3)
144 (2.6)
<0.001
Hospital outcomes
Length of stay, days (IQR)
8.6 (3.9–18.6)
3.0 (1.8–6.5)
<0.001
ICU admission
1,421 (36.5)
961 (17.6)
<0.001
In-hospital mortality
828 (21.0)
190 (3.8)
<0.001
Abbreviations: ARDS = acute respiratory distress syndrome; CHF = congestive heart
failure; COPD = chronic obstructive pulmonary disease; COVID-19 = coronavirus disease
2019; DIC = disseminated intravascular coagulation; ICU = intensive care unit; IQR = interquartile
range; MI = myocardial infarction.
* Data on race or ethnicity were missing for 199 (5.0%) patients with COVID-19 and
160 (2.9%) patients with influenza; data on underlying medical conditions were missing
for 163 (4.1%) patients with COVID-19 and 75 (1.4%) patients with influenza; and data
on ICU admission was missing for 49 (1.2%) patients with COVID-19. P-values were calculated
from Chi-squared or Fisher’s exact test for categorical variables and Wilcoxon rank
sum test for continuous variables.
† Among patients with COVID-19, non-Hispanic Other included 22 patients with multiple
races documented, 22 American Indians or Alaska Natives, 29 Asians, and 14 Native
Hawaiians or other Pacific Islanders. Among patients with influenza, non-Hispanic
Other included 47 patients with multiple races documented, 34 American Indians or
Alaska Natives, 29 Asians, and 40 Native Hawaiians or other Pacific Islanders.
§ Coding of underlying medical conditions was based on International Classification
of Diseases, Tenth Revision,
Clinical Modification, (ICD-10-CM) codes and grouping into categories was based primarily
on established categorizations from the CDC Hospitalized Adult Influenza Vaccine Effectiveness
Network.
¶ Excluding hypertension only.
** Complications are not mutually exclusive. Acute complications primarily identified
using a list of ICD-10-CM codes published by Chow EJ, Rolfes MA, O'Halloran A, et
al. (https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2762991). Complications
assessed but not included in Chow et al. include pressure ulcers (ICD-10-CM code L89*)
and dialysis initiation (ICD-10-CM codes Z49, Z99.2, Z95.3, and Z91.15 and Current
Procedural Terminology codes 90935, 90937, 90940, 90945, 90947, 90999, 0505F, 4045F,
36800, 36810, and 36816).
†† Denominator restricted to patients with the underlying medical condition related
to the complication (asthma, COPD, or diabetes mellitus).
§§ Indication of dialysis during hospitalization without indication of dialysis within
the past year.
¶¶ Other rare complications reported in <1% of patients with COVID-19 included acute
pericarditis (seven, 0.2%), immune thrombocytopenic purpura (seven, 0.2%), Guillain-Barre
Syndrome (six, 0.2%), encephalitis (seven, 0.2%), acute disseminated encephalomyelitis
and encephalomyelitis (one, <0.1%), thyrotoxicosis (19, 0.5%), hyperglycemic hyperosmolar
syndrome (14, 0.4%), acute pancreatitis (16, 0.4%), rhabdomyolysis (83, 2.1%), and
autoimmune hemolytic anemia (one, <0.1%).
Among patients with COVID-19, 76.8% had respiratory complications, including pneumonia
(70.1%), respiratory failure (46.5%), and ARDS (9.3%). Nonrespiratory complications
were frequent, including renal (39.6%), cardiovascular (13.1%), hematologic (6.2%),
and neurologic complications (4.1%), as well as sepsis (24.9%) and bacteremia (4.7%);
24.1% of COVID-19 patients had complications involving three or more organ systems.
Among COVID-19 patients, nine complications were more prevalent among racial and ethnic
minority patients, including respiratory, neurologic, and renal complications, even
after adjustment for age and underlying medical conditions (Table 2).
TABLE 2
Proportions and adjusted relative risk of selected COVID-19 respiratory and nonrespiratory
complications,* by race/ethnicity
†
— Veterans Health Administration, United States, March 1–May 31, 2020
Complication
White, non-Hispanic
(N = 1,515)
Black or African American, non-Hispanic
(N = 1,811)
Other race, non-Hispanic§
(N = 87)
Hispanic or Latino
(N = 336)
P-value**
No. (%)
No. (%)
aRR (95% CI)¶
No. (%)
aRR (95% CI)¶
No. (%)
aRR (95% CI)¶
Pneumonia
967 (63.8)
1,322 (73.0)
1.15 (1.10–1.21)
64 (73.6)
1.15 (1.01–1.31)
257 (76.5)
1.21 (1.13–1.31)
<0.001
Respiratory failure
656 (43.3)
860 (47.5)
1.14 (1.06–1.23)
48 (55.2)
1.30 (1.08–1.58)
158 (47.0)
1.13 (0.99–1.28)
0.03
ARDS
118 (7.8)
177 (9.8)
1.25 (1.00–1.57)
15 (17.2)
2.06 (1.24–3.43)
38 (11.3)
1.32 (0.92–1.91)
0.01
Hypertensive crisis
11 (0.7)
33 (1.8)
2.27 (1.13–4.54)
3 (3.4)
4.03 (1.14–14.21)
2 (0.6)
0.87 (0.19–3.90)
0.01
Cerebral ischemia/infarction
29 (1.9)
69 (3.8)
2.42 (1.57–3.74)
2 (2.3)
1.34 (0.33–5.50)
17 (5.1)
3.44 (1.92–6.18)
<0.01
Intracranial hemorrhage
6 (0.4)
15 (0.8)
2.45 (0.88–6.80)
3 (3.4)
10.36 (2.54–42.31)
3 (0.9)
2.69 (0.64–11.25)
0.02
Acute kidney failure
483 (31.9)
845 (46.7)
1.40 (1.28–1.53)
36 (41.4)
1.29 (1.01–1.66)
108 (32.1)
1.06 (0.89–1.26)
<0.001
Dialysis initiation
21 (1.4)
83 (4.7)
2.92 (1.81–4.71)
2 (2.4)
1.47 (0.35–6.16)
9 (2.9)
2.09 (0.97–4.52)
<0.001
Sepsis
306 (20.2)
496 (27.4)
1.42 (1.25–1.61)
29 (33.3)
1.71 (1.25–2.34)
91 (27.1)
1.40 (1.14–1.73)
<0.001
Abbreviations: ARDS = acute respiratory distress syndrome; aRR = adjusted risk ratio;
CI = confidence interval; COVID-19 = coronavirus disease 2019.
* Complications are not mutually exclusive. Other complications assessed but not statistically
different (p-value >0.05) across strata of race/ethnicity included pneumothorax, asthma
and chronic obstructive pulmonary disease exacerbation, acute myocardial infarction/unstable
angina, acute congestive heart failure, acute myocarditis, cardiogenic shock, deep
vein thrombosis, pulmonary embolism, disseminated intravascular coagulation, diabetic
ketoacidosis, acute hepatitis/liver failure, bacteremia, and pressure ulcers.
† Data on race/ethnicity were missing for 199 (5.0%) of COVID-19 patients and were
excluded from the race/ethnicity stratification.
§ Other, non-Hispanic category included 22 patients with multiple races documented,
22 American Indians or Alaska Natives, 29 Asians, and 14 Native Hawaiians or other
Pacific Islanders.
¶ Separate log-binomial models were run to estimate aRRs for each complication. Pneumonia,
respiratory failure, and ARDS models adjusted for age, COPD, asthma, and other lung
diseases; hypertensive crisis model adjusted for age, hypertension, heart disease,
and heart failure; cerebral ischemia/infarction and intracranial hemorrhage models
controlled for age, underlying cerebrovascular diseases, neurologic/musculoskeletal
conditions, heart disease, and heart failure; acute kidney failure and dialysis models
controlled for age, underlying renal disease, diabetes mellitus, and hypertension.
** P-values calculated from Chi-squared or Fisher’s exact test to compare frequencies
of complications among strata of race/ethnicity.
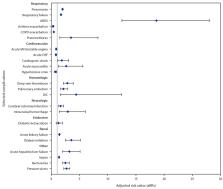
Compared with patients with influenza, patients with COVID-19 had two times the risk
for pneumonia; 1.7 times the risk for respiratory failure; 19 times the risk for ARDS;
3.5 times the risk for pneumothorax; and statistically significantly increased risks
for cardiogenic shock, myocarditis, deep vein thrombosis, pulmonary embolism, disseminated
intravascular coagulation, cerebral ischemia or infarction, intracranial hemorrhage,
acute kidney failure, dialysis initiation, acute hepatitis or liver failure, sepsis,
bacteremia, and pressure ulcers (Figure). Patients with COVID-19 had a lower risk
for five complications (asthma exacerbation, COPD exacerbation, acute myocardial infarction
(MI) or unstable angina, acute congestive heart failure (CHF), and hypertensive crisis),
although acute MI or unstable angina, acute CHF, and hypertensive crisis were not
statistically significant when restricting to patients diagnosed during the same seasonal
months.
FIGURE
Adjusted relative risk* for selected acute respiratory and nonrespiratory complications
in hospitalized patients with COVID-19 (March 1– May 31, 2020), compared with historically
hospitalized patients with influenza (October 1, 2018–February 1, 2020) — Veterans
Health Administration, United States
†
,
§
,
¶
Abbreviations: ARDS = acute respiratory distress syndrome; CHF = congestive heart
failure; COPD = chronic obstructive pulmonary disease; DIC = disseminated intravascular
coagulation; MI = myocardial infarction.
* 95% confidence intervals (CIs) indicated with error bars.
† When restricted to patients with influenza during the same seasonal months (March–May),
aRRs and 95% CIs for acute MI or unstable angina, acute CHF, and hypertensive crisis
were 0.90 (0.74–1.11), 1.03 (0.82–1.28), and 0.75 (0.44–1.29), respectively.
§ Dialysis during hospitalization was identified using International Classification
of Diseases, Tenth Revision, Clinical Modification and current procedural terminology
codes, and new initiation of dialysis was determined by excluding patients with indication
of dialysis within the past year.
¶ Separate crude and adjusted log-binomial models were run for each complication (which
were not mutually exclusive). All adjusted models adjusted for age, sex, race/ethnicity,
and outcome-specific underlying conditions. Specifically, respiratory complication
models controlled for COPD, asthma, and other lung diseases; neurologic complication
models controlled for underlying cerebrovascular diseases, neurological/musculoskeletal
conditions, heart disease, and heart failure; cardiovascular and hematologic condition
models controlled for heart disease, heart failure, renal conditions, diabetes mellitus,
and extreme obesity; the acute kidney failure model controlled for underlying renal
disease, diabetes mellitus, and hypertension. Complications related to the worsening
of a chronic medical condition were restricted to those patients with that underlying
medical condition.
The figure is a forest plot showing the adjusted relative risk for selected acute
respiratory and nonrespiratory complications in hospitalized patients with COVID-19
(March 1–May 31, 2020), compared with historically hospitalized patients with influenza
(October 1, 2018–February 1, 2020) among U.S. patients in the Veterans Health Administration
system.
Discussion
Findings from a large, national cohort of patients hospitalized within the VHA illustrate
the increased risk for complications involving multiple organ systems among patients
with COVID-19 compared with those with influenza, as well as racial/ethnic disparities
in COVID-19–associated complications. Compared with patients with influenza, those
with COVID-19 had a more than five times higher risk for in-hospital death and approximately
double the ICU admission risk and hospital length of stay, and were at higher risk
for 17 acute respiratory, cardiovascular, hematologic, neurologic, renal and other
complications. Racial and ethnic disparities in the percentage of complications among
patients with COVID-19 was found for respiratory, neurologic, and renal complications,
as well as for sepsis.
Persons from racial and ethnic minority groups are increasingly recognized as having
higher rates of COVID-19, associated hospitalizations, and increased risk for severe
in-hospital outcomes (
4
,
5
). Although previous analysis of VHA data found no differences in COVID-19 mortality
by race/ethnicity (
4
), in this analysis, Black, Hispanic, and non-Hispanic patients of other races had
higher risks for sepsis and respiratory, neurologic, and renal complications than
did White patients. The disparities in acute complications among racial and ethnic
minority groups could not solely be accounted for by differences in underlying medical
conditions or age and might be affected by social, environmental, economic, and structural
inequities.
¶
Elucidation of the reasons for these disparities is urgently needed to advance health
equity for all persons.
The risk for respiratory complications was high, consistent with current knowledge
of SARS-CoV-2 and influenza pathogenesis (
1
,
6
). Notably, compared with patients with influenza, patients with COVID-19 had two
times the risk for pneumonia, 1.7 times the risk for respiratory failure, 19 times
the risk for ARDS, and 3.5 times the risk for pneumothorax, underscoring the severity
of COVID-19 respiratory illness relative to that of influenza. Conversely, the risk
for asthma and COPD exacerbations was approximately three times lower among patients
with COVID-19 than among those with influenza.
The risk for certain acute nonrespiratory complications was also high, including the
risk for sepsis and renal and cardiovascular complications. Patients with COVID-19
were at increased risk for acute kidney failure requiring dialysis than were patients
with influenza, consistent with previous evidence of influenza- (
2
) and COVID-19–associated (
7
) acute kidney failure. The frequent occurrence and increased risk for sepsis among
patients with COVID-19 is consistent with reports of dysregulated immune response
in these patients (
8
). The distribution of cardiovascular complications differed between patients with
influenza and those with COVID-19; patients with COVID-19 experienced lower risk for
acute MI, unstable angina, and acute CHF but higher risk for acute myocarditis and
cardiogenic shock. There were no significant differences in occurrence of acute MI,
unstable angina, and CHF among patients with COVID-19 or influenza diagnosed during
the same months, suggesting potential confounding by seasonal variations in cardiovascular
disease.
Other less common (<10%), but often severe complications included hematologic and
neurologic complications, bacteremia, and pressure ulcers. Whereas other viruses,
like influenza, might cause proinflammatory cytokines and clot formation (
6
), the findings from this study suggest that hematologic complications are a much
more frequent complication of COVID-19, consistent with previous reports of COVID-19–related
thromboembolic events (
1
,
9
). A New York City study reported that the odds of stroke were 7.6 times higher among
COVID-19 patients than among those with influenza (
10
), which is consistent with the present findings of a twofold increase in the risk
for cerebral ischemia or infarction. Patients with COVID-19 might be at increased
risk for pressure ulcers related to prolonged hospitalizations, prone positioning,
or both.
The findings in this report are subject to at least six limitations. First, administrative
codes might have limited sensitivity and specificity for capturing conditions and
might misclassify chronic conditions as acute. Extreme obesity was defined based solely
on ICD-10-CM codes and not body mass index, resulting in potential misclassification
and residual confounding. Second, clinician-ordered testing could potentially underestimate
some complications in patients with less typical respiratory symptoms. Third, the
analysis of racial differences was limited by the small sample size within the non-Hispanic
Other race group. Fourth, the generalizability of results might be limited by the
diversity and moderate severity among adults of the predominant circulating influenza
type/subtype during the period of this analysis (A H3N2 in 2018–2019 and A H1N1 and
B in 2019–2020).** Fifth, influenza vaccination or treatments for COVID-19 or influenza
that might affect these outcomes were not examined. Finally, this analysis did not
adjust for region or facility size or type, and further research is warranted to assess
the impact of these factors on the risk for COVID-19 complications.
Hospitalized adult VHA patients with COVID-19 experienced a higher risk for respiratory
and nonrespiratory complications and death than did hospitalized patients with influenza.
Disparities by race/ethnicity in experiencing sepsis and respiratory, neurologic,
and renal complications, even after adjustment for age and underlying medical conditions,
provide further evidence that racial and ethnic minority groups are disproportionally
affected by COVID-19. Clinicians should be vigilant for symptoms and signs of a spectrum
of complications among hospitalized patients with COVID-19 so that interventions can
be instituted to improve outcomes and reduce long-term disability.
Summary
What is already known about this topic?
Patients hospitalized with COVID-19 are reported to be at risk for respiratory and
nonrespiratory complications.
What is added by this report?
Hospitalized patients with COVID-19 in the Veterans Health Administration had a more
than five times higher risk for in-hospital death and increased risk for 17 respiratory
and nonrespiratory complications than did hospitalized patients with influenza. The
risks for sepsis and respiratory, neurologic, and renal complications of COVID-19
were higher among non-Hispanic Black or African American and Hispanic patients than
among non-Hispanic White patients.
What are the implications for public health practice?
Compared with influenza, COVID-19 is associated with increased risk for most respiratory
and nonrespiratory complications. Certain racial and ethnic minority groups are disproportionally
affected by COVID-19.
Related collections
Most cited references10
- Record: found
- Abstract: found
- Article: not found
Extrapulmonary manifestations of COVID-19
Aakriti Gupta, Mahesh Madhavan, Kartik Sehgal … (2020)
- Record: found
- Abstract: found
- Article: not found
COVID-19 and its implications for thrombosis and anticoagulation
Jean M Connors, Jerrold Levy (2020)
- Record: found
- Abstract: found
- Article: not found
SARS-CoV-2 and viral sepsis: observations and hypotheses
Hui LI, Liang Liu, Dingyu Zhang … (2020)