- Record: found
- Abstract: found
- Article: found
Protein losing enteropathy: comprehensive review of the mechanistic association with clinical and subclinical disease states
Read this article at
Abstract
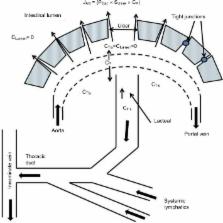
Protein losing enteropathy (PLE) has been associated with more than 60 different conditions, including nearly all gastrointestinal diseases (Crohn’s disease, celiac, Whipple’s, intestinal infections, and so on) and a large number of non-gut conditions (cardiac and liver disease, lupus, sarcoidosis, and so on). This review presents the first attempt to quantitatively understand the magnitude of the PLE in relation to the associated pathology for three different disease categories: 1) increased lymphatic pressure (e.g., lymphangiectasis); 2) diseases with mucosal erosions (e.g., Crohn’s disease); and 3) diseases without mucosal erosions (e.g., celiac disease). The PLE with lymphangiectasis results from rupture of the mucosal lymphatics, with retrograde drainage of systemic lymph into the intestinal lumen with the resultant loss of CD4 T cells, which is diagnostic. Mucosal erosion PLE results from macroscopic breakdown of the mucosal barrier, with the epithelial capillaries becoming the rate-limiting factor in albumin loss. The equation derived to describe the relationship between the reduction in serum albumin (C P) and PLE indicates that gastrointestinal albumin clearance must increase by at least 17 times normal to reduce the C P by half. The strengths and limitations of the two quantitative measures of PLE ( 51Cr-albumin or α 1-antitrypsin [αAT] clearance) are reviewed. αAT provides a simple quantitative diagnostic test that is probably underused clinically. The strong, unexplained correlation between minor decreases in C P and subsequent mortality in seemingly healthy individuals raises the question of whether subclinical PLE could account for the decreased C P and, if so, could the mechanism responsible for PLE play a role in the increased mortality? A large-scale study correlating αAT clearance with serum albumin concentrations will be required in order to determine the role of PLE in the regulation of the serum albumin concentration of seemingly healthy subjects.
Related collections
Most cited references123
- Record: found
- Abstract: found
- Article: not found
Loss of Infliximab Into Feces Is Associated With Lack of Response to Therapy in Patients With Severe Ulcerative Colitis.
- Record: found
- Abstract: found
- Article: not found
MYO5B mutations cause microvillus inclusion disease and disrupt epithelial cell polarity.
- Record: found
- Abstract: found
- Article: not found