- Record: found
- Abstract: found
- Article: not found
The health effects of vitamin D supplementation: evidence from human studies
Read this article at
Abstract
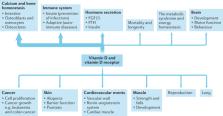
Vitamin D supplementation can prevent and cure nutritional rickets in infants and children. Preclinical and observational data suggest that the vitamin D endocrine system has a wide spectrum of skeletal and extra-skeletal activities. There is consensus that severe vitamin D deficiency (serum 25-hydroxyvitamin D (25OHD) concentration <30 nmol/l) should be corrected, whereas most guidelines recommend serum 25OHD concentrations of >50 nmol/l for optimal bone health in older adults. However, the causal link between vitamin D and many extra-skeletal outcomes remains unclear. The VITAL, ViDA and D2d randomized clinical trials (combined number of participants >30,000) indicated that vitamin D supplementation of vitamin D-replete adults (baseline serum 25OHD >50 nmol/l) does not prevent cancer, cardiovascular events, falls or progression to type 2 diabetes mellitus. Post hoc analysis has suggested some extra-skeletal benefits for individuals with vitamin D deficiency. Over 60 Mendelian randomization studies, designed to minimize bias from confounding, have evaluated the consequences of lifelong genetically lowered serum 25OHD concentrations on various outcomes and most studies have found null effects. Four Mendelian randomization studies found an increased risk of multiple sclerosis in individuals with genetically lowered serum 25OHD concentrations. In conclusion, supplementation of vitamin D-replete individuals does not provide demonstrable health benefits. This conclusion does not contradict older guidelines that severe vitamin D deficiency should be prevented or corrected.
Abstract
This Review highlights results from large randomized clinical trials performed during the period 2017–2020 and Mendelian randomization studies on vitamin D levels. Together, findings indicate that vitamin D supplementation alone in vitamin D-replete adults does not provide health benefits; however, vitamin D deficiency should always be corrected.
Key points
-
Vitamin D and calcium supplementation can cure nutritional rickets and can modestly decrease the risk of major fractures in older adults with poor vitamin D status or calcium intake.
-
Large supplementation trials recruiting vitamin D-replete adults (serum 25OHD concentration >50 nmol/l) have demonstrated no effects on the incidence of cancer, cardiovascular events or type 2 diabetes mellitus (T2DM) and no benefits in terms of bone density and the risk of falls.
-
Post-hoc analysis of large supplementation trials has suggested that supplementation of individuals with vitamin D deficiency modestly delays age-related bone loss and progression to T2DM, and improves lung function.
-
A meta-analysis suggested that vitamin D supplementation results in a modest decrease in cancer mortality.
-
Over 60 Mendelian randomization studies have examined causal links between genetically lower vitamin D levels and health outcomes; most studies generated null effects except four studies that demonstrated an increased risk of multiple sclerosis.
-
In conclusion, supplementation of vitamin D-replete individuals does not generate overall health benefits; however, correction of severe vitamin D deficiency remains essential.
Related collections
Most cited references123
- Record: found
- Abstract: found
- Article: not found
Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline.
- Record: found
- Abstract: found
- Article: not found
Vitamin D Supplements and Prevention of Cancer and Cardiovascular Disease
- Record: found
- Abstract: found
- Article: not found
