- Record: found
- Abstract: found
- Article: found
A role for CBFβ in maintaining the metastatic phenotype of breast cancer cells
Read this article at
Abstract
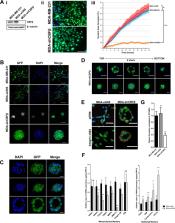
Epithelial to mesenchymal transition (EMT) is a dynamic process that drives cancer cell plasticity and is thought to play a major role in metastasis. Here we show, using MDA-MB-231 cells as a model, that the plasticity of at least some metastatic breast cancer cells is dependent on the transcriptional co-regulator CBFβ. We demonstrate that CBFβ is essential to maintain the mesenchymal phenotype of triple-negative breast cancer cells and that CBFβ-depleted cells undergo a mesenchymal to epithelial transition (MET) and re-organise into acini-like structures, reminiscent of those formed by epithelial breast cells. We subsequently show, using an inducible CBFβ system, that the MET can be reversed, thus demonstrating the plasticity of CBFβ-mediated EMT. Moreover, the MET can be reversed by expression of the EMT transcription factor Slug whose expression is dependent on CBFβ. Finally, we demonstrate that loss of CBFβ inhibits the ability of metastatic breast cancer cells to invade bone cell cultures and suppresses their ability to form bone metastases in vivo. Together our findings demonstrate that CBFβ can determine the plasticity of the metastatic cancer cell phenotype, suggesting that its regulation in different micro-environments may play a key role in the establishment of metastatic tumours.
Related collections
Most cited references21
- Record: found
- Abstract: found
- Article: not found
Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures.
- Record: found
- Abstract: found
- Article: not found
Regulation of breast cancer stem cell activity by signaling through the Notch4 receptor.
- Record: found
- Abstract: found
- Article: not found