- Record: found
- Abstract: found
- Article: found
Gene interfered-ferroptosis therapy for cancers
Read this article at
Abstract
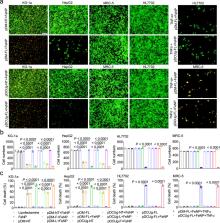
Although some effective therapies have been available for cancer, it still poses a great threat to human health and life due to its drug resistance and low response in patients. Here, we develop a ferroptosis-based therapy by combining iron nanoparticles and cancer-specific gene interference. The expression of two iron metabolic genes ( FPN and LCN2) was selectively knocked down in cancer cells by Cas13a or microRNA controlled by a NF-κB-specific promoter. Cells were simultaneously treated by iron nanoparticles. As a result, a significant ferroptosis was induced in a wide variety of cancer cells. However, the same treatment had little effect on normal cells. By transferring genes with adeno-associated virus and iron nanoparticles, the significant tumor growth inhibition and durable cure were obtained in mice with the therapy. In this work, we thus show a cancer therapy based on gene interference-enhanced ferroptosis.
Abstract
Improved therapeutic strategies are needed as drug resistance limits the therapeutic efficacy of several clinically approved cancer therapeutics. Here, the authors report a ferroptosis-based therapy using a combination of iron nanoparticles with gene interference to knockdown iron metabolic genes, FPN and LCN2.
Related collections
Most cited references60
- Record: found
- Abstract: found
- Article: not found
Ferroptosis: an iron-dependent form of nonapoptotic cell death.
- Record: found
- Abstract: found
- Article: not found
Intrinsic peroxidase-like activity of ferromagnetic nanoparticles.

- Record: found
- Abstract: found
- Article: found