- Record: found
- Abstract: found
- Article: found
Mineral and Electrolyte Disorders With SGLT2i Therapy
Read this article at
ABSTRACT
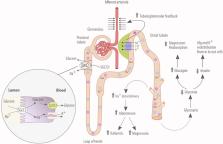
The newly developed sodium‐glucose cotransporter 2 inhibitors (SGLT2is) effectively modulate glucose metabolism in diabetes. Although clinical data suggest that SGLT2is (empagliflozin, dapagliflozin, ertugliflozin, canagliflozin, ipragliflozin) are safe and protect against renal and cardiovascular events, very little attention has been dedicated to the effects of these compounds on different electrolytes. As with other antidiabetic compounds, some effects on water and electrolytes balance have been documented. Although the natriuretic effect and osmotic diuresis are expected with SGLT2is, these compounds may also modulate urinary potassium, magnesium, phosphate, and calcium excretion. Notably, they have had no effect on plasma sodium levels and promoted only small increases in serum potassium and magnesium concentrations in clinical trials. Moreover, SGLT2is may induce an increase in serum phosphate, FGF‐23, and PTH; reduce 1,25‐dihydroxyvitamin D; and generate normal serum calcium. Some published and preliminary reports, as well as unconfirmed reports have suggested an association with bone fractures. Some homeostasis perturbations are transient, whereas others may persist, suggesting that the administration of SGLT2is may affect electrolyte balances in exposed subjects. Although current evidence supports their safety, additional efforts are needed to elucidate the long‐term impact of these compounds on chronic kidney disease, mineral metabolism, and bone health. Indeed, the limited follow‐up studies and the heterogeneity of the case‐mix of different randomized controlled trials preclude a definitive answer on the impact of these compounds on long‐term outcomes such as the risk of bone fracture. Here we review the current understanding of the mechanisms involved in electrolyte handling and the available data on the clinical implications of electrolytes and mineral metabolism perturbations induced by SGLT2i administration. © 2019 The Authors. JBMR Plus published by Wiley Periodicals, Inc. on behalf of American Society for Bone and Mineral Research.
Related collections
Most cited references44
- Record: found
- Abstract: found
- Article: not found
Dapagliflozin maintains glycaemic control while reducing weight and body fat mass over 2 years in patients with type 2 diabetes mellitus inadequately controlled on metformin.
- Record: found
- Abstract: found
- Article: not found