- Record: found
- Abstract: found
- Article: found
LncRNA XIST acts as a tumor suppressor in prostate cancer through sponging miR-23a to modulate RKIP expression

Read this article at
Abstract
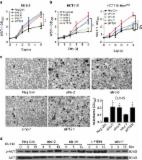
Accumulating evidences have indicated that aberrant expression of long non-coding RNAs (LncRNAs) is tightly associated with cancer development. Previous studies have reported that lncRNA XIST regulates tumor malignancies in several cancers. However, the underlying mechanism of XIST in prostate cancer remains unclear. In the current study, we found that XIST was down-regulated in prostate cancer specimens and cell lines. Low expression of XIST was correlated with poor prognosis and advanced tumor stage in prostate cancer patients. In gain and loss of function assays, we confirmed that XIST suppressed cellular proliferation and metastasis in prostate cancer both in vitro and in vivo. Furthermore, we found that XIST negatively regulates the expression of miR-23a and subsequently promotes RKIP expression at post-transcriptional level. Consequently, we investigated the correlation between XIST and miR-23a, and identified miR-23a as a direct target of XIST. In addition, over-expression of miR-23a efficiently abrogated the up-regulation of RKIP induced by XIST, suggesting that XIST positively regulates the expression of RKIP by competitively binding to miR-23a. Taken together, our study indicated that lncRNA XIST acts as a tumor suppressor in prostate cancer, and this regulatory effect of XIST will shed new light on epigenetic diagnostics and therapeutics in prostate cancer.
Related collections
Most cited references26
- Record: found
- Abstract: found
- Article: not found
The Xist lncRNA exploits three-dimensional genome architecture to spread across the X chromosome.
- Record: found
- Abstract: found
- Article: found
Integrative analyses reveal a long noncoding RNA-mediated sponge regulatory network in prostate cancer
- Record: found
- Abstract: found
- Article: not found