- Record: found
- Abstract: found
- Article: not found
A Synthesis and Crystal Chemical Study of the Fast Ion Conductor Li 7–3 x Ga x La 3 Zr 2O 12 with x = 0.08 to 0.84
Read this article at
Abstract

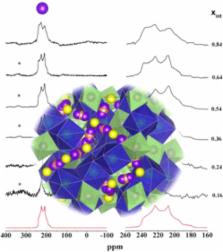
Fast-conducting phase-pure cubic Ga-bearing Li 7La 3Zr 2O 12 was obtained using solid-state synthesis methods with 0.08 to 0.52 Ga 3+ pfu in the garnet. An upper limit of 0.72 Ga 3+ pfu in garnet was obtained, but the synthesis was accompanied by small amounts of La 2Zr 2O 12 and LiGaO 3. The synthetic products were characterized by X-ray powder diffraction, electron microprobe and SEM analyses, ICP-OES measurements, and 71Ga MAS NMR spectroscopy. The unit-cell parameter, a 0, of the various garnets does not vary significantly as a function of Ga 3+ content, with a value of about 12.984(4) Å. Full chemical analyses for the solid solutions were obtained giving: Li 7.08Ga 0.06La 2.93Zr 2.02O 12, Li 6.50Ga 0.15La 2.96Zr 2.05O 12, Li 6.48Ga 0.23La 2.93Zr 2.04O 12, Li 5.93Ga 0.36La 2.94Zr 2.01O 12, Li 5.38Ga 0.53La 2.96Zr 1.99O 12, Li 4.82Ga 0.60La 2.96Zr 2.00O 12, and Li 4.53Ga 0.72La 2.94Zr 1.98O 12. The NMR spectra are interpreted as indicating that Ga 3+ mainly occurs in a distorted 4-fold coordinated environment that probably corresponds to the general 96 h crystallographic site of garnet.
Abstract
Fast-conducting cubic Ga-bearing Li 7La 3Zr 2O 12 (LLZO) has been synthesized using solid-state methods with 0.08 to 0.52 Ga 3+ pfu in the garnet. An upper limit of 0.72 Ga 3+ pfu in the garnet was obtained, but the synthesis product contained small amounts of La 2Zr 2O 12 and LiGaO 3. The NMR spectra are interpreted as indicating that Ga 3+ mainly occurs in a distorted 4-fold coordinated environment that corresponds to the “non-standard” general 96 h crystallographic site.
Related collections
Most cited references7
- Record: found
- Abstract: found
- Article: not found
Structure and dynamics of the fast lithium ion conductor "Li7La3Zr2O12".
- Record: found
- Abstract: found
- Article: found
Short to long-range charge-transfer excitations in the zincbacteriochlorin-bacteriochlorin complex: a Bethe-Salpeter study
- Record: found
- Abstract: found
- Article: not found
