- Record: found
- Abstract: found
- Article: found
CCR4 Controls the Suppressive Effects of Regulatory T Cells on Early and Late Events during Severe Sepsis
Read this article at
Abstract
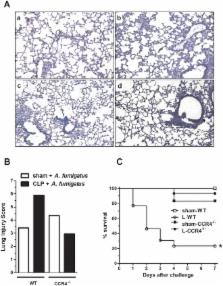
Sepsis is a deadly disease characterized by an overwhelming release of inflammatory mediators and the activation of different types of cells. This altered state of cell activation, termed leukocyte reprogramming, contributes to patient outcome. However, the understanding of the process underlying sepsis and the role of regulatory T cells (Tregs) in sepsis remains to be elucidated. In this study, we investigated the role of CCR4, the CCL17/CCL22 chemokine receptor, in the innate and acquired immune responses during severe sepsis and the role of Tregs in effecting the outcome. In contrast with wild-type (WT) mice subjected to cecal ligation and puncture (CLP) sepsis, CCR4-deficient (CCR4 -/-) septic mice presented an increased survival rate, significant neutrophil migration toward the infection site, a low bacterial count in the peritoneum, and reduced lung inflammation and serum cytokine levels. Thus, a better early host response may favor an adequate long-term response. Consequently, the CCR4 -/- septic mice were not susceptible to secondary fungal infection, in contrast with the WT septic mice. Furthermore, Tregs cells from the CCR4 -/- septic mice showed reduced suppressive effects on neutrophil migration (both in vivo and in vitro), lymphocyte proliferation and ROS production from activated neutrophils, in contrast with what was observed for Tregs from the WT septic mice. These data show that CCR4 is involved in immunosuppression after severe sepsis and suggest that CCR4 + Tregs negatively modulate the short and long-term immune responses.
Related collections
Most cited references57
- Record: found
- Abstract: found
- Article: not found
Unique Chemotactic Response Profile and Specific Expression of Chemokine Receptors Ccr4 and Ccr8 by Cd4+Cd25+ Regulatory T Cells
- Record: found
- Abstract: found
- Article: not found
Tumor-infiltrating monocytic myeloid-derived suppressor cells mediate CCR5-dependent recruitment of regulatory T cells favoring tumor growth.
- Record: found
- Abstract: found
- Article: not found