- Record: found
- Abstract: found
- Article: found
PRG-1 prevents neonatal stimuli-induced persistent hyperalgesia and memory dysfunction via NSF/Glu/GluR2 signaling

Read this article at
Summary
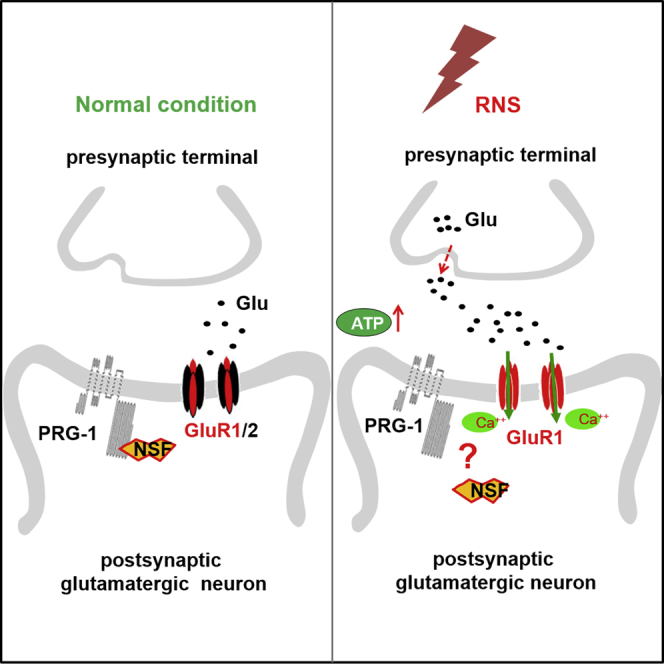
Neonatal repetitive noxious stimuli (RNS) has been shown to cause long-term harmful effects on nociceptive processing, learning, and memory which persist until adulthood. Plasticity-related gene 1 (PRG-1) regulates synaptic plasticity and functional reorganization in the brain during neuronal development. In this study, neonatal RNS rats were established by repetitive needle pricks to neonatal rats on all four feet to model repetitive pain exposure in infants. Neonatal RNS caused thermal hyperalgesia, mechanical allodynia, learning, and memory impairments which manifested in young rats and persisted until adulthood. Hippocampal PRG-1/N-ethylmaleimide sensitive fusion protein (NSF) interaction was determined to be responsible for the RNS-induced impairment via enhanced extracellular glutamate release and AMPAR GluR2 trafficking deficiency in a cell-autonomous manner. These pathways likely act synergistically to cause changes in dendritic spine density. Our findings suggest that PRG-1 prevents the RNS-induced hyperalgesia, learning, and memory impairment by regulating synaptic plasticity via NSF/Glu/GluR2 signaling.
Graphical abstract
Highlights
Abstract
Behavioral neuroscience; Molecular neuroscience; Developmental neuroscience; Cellular neuroscience
Related collections
Most cited references68
- Record: found
- Abstract: found
- Article: not found
Morris water maze: procedures for assessing spatial and related forms of learning and memory.
- Record: found
- Abstract: not found
- Article: not found
Ethical guidelines for investigations of experimental pain in conscious animals
- Record: found
- Abstract: found
- Article: not found