- Record: found
- Abstract: found
- Article: found
Icariin regulates miR-23a-3p-mediated osteogenic differentiation of BMSCs via BMP-2/Smad5/Runx2 and WNT/β-catenin pathways in osteonecrosis of the femoral head
Read this article at
Abstract
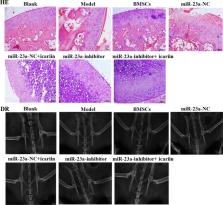
Icariin is commonly used for the clinical treatment of osteonecrosis of the femoral head (ONFH). miR-23a-3p plays a vital role in regulating the osteogenic differentiation of bone marrow-derived mesenchymal stem cells (BMSCs). The present study aimed to investigate the roles of icariin and miR-23a-3p in the osteogenic differentiation of BMSCs and an ONFH model. BMSCs were isolated and cultured in vitro using icariin-containing serum at various concentrations, and BMSCs were also transfected with a miR-23a inhibitor. The alkaline phosphatase (ALP) activity and cell viability as well as BMP-2/Smad5/Runx2 and WNT/β-catenin pathway-related mRNA and protein expression were measured in BMSCs. Additionally, a dual-luciferase reporter assay and pathway inhibitors were used to verify the relationship of icariin treatment/miR-23a and the above pathways. An ONFH rat model was established in vivo, and a 28-day gavage treatment and lentivirus transfection of miR-23a-3p inhibitor were performed. Then, bone biochemical markers (ELISA kits) in serum, femoral head (HE staining and Digital Radiography, DR) and the above pathway-related proteins were detected. Our results revealed that icariin treatment/miR-23a knockdown promoted BMSC viability and osteogenic differentiation as well as increased the mRNA and protein expression of BMP-2, BMP-4, Runx2, p-Smad5, Wnt1 and β-catenin in BMSCs and ONFH model rats. In addition, icariin treatment/miR-23a knockdown increased bone biochemical markers (ACP-5, BAP, NTXI, CTXI and OC) and improved ONFH in ONFH model rats. In addition, a dual-luciferase reporter assay verified that Runx2 was a direct target of miR-23a-3p. These data indicated that icariin promotes BMSC viability and osteogenic differentiation as well as improves ONFH by decreasing miR-23a-3p levels and regulating the BMP-2/Smad5/Runx2 and WNT/β-catenin pathways.
Related collections
Most cited references51
- Record: found
- Abstract: found
- Article: not found
BMSC-derived exosomes carrying microRNA-122-5p promote proliferation of osteoblasts in osteonecrosis of the femoral head.
- Record: found
- Abstract: found
- Article: found
miRNAs in bone tissue correlate to bone mineral density and circulating miRNAs are gender independent in osteoporotic patients

- Record: found
- Abstract: found
- Article: found