- Record: found
- Abstract: found
- Article: found
Yellow Fever: Integrating Current Knowledge with Technological Innovations to Identify Strategies for Controlling a Re-Emerging Virus

Read this article at
Abstract
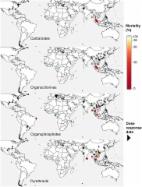
Yellow fever virus (YFV) represents a re-emerging zoonotic pathogen, transmitted by mosquito vectors to humans from primate reservoirs. Sporadic outbreaks of YFV occur in endemic tropical regions, causing a viral hemorrhagic fever (VHF) associated with high mortality rates. Despite a highly effective vaccine, no antiviral treatments currently exist. Therefore, YFV represents a neglected tropical disease and is chronically understudied, with many aspects of YFV biology incompletely defined including host range, host–virus interactions and correlates of host immunity and pathogenicity. In this article, we review the current state of YFV research, focusing on the viral lifecycle, host responses to infection, species tropism and the success and associated limitations of the YFV-17D vaccine. In addition, we highlight the current lack of available treatments and use publicly available sequence and structural data to assess global patterns of YFV sequence diversity and identify potential drug targets. Finally, we discuss how technological advances, including real-time epidemiological monitoring of outbreaks using next-generation sequencing and CRISPR/Cas9 modification of vector species, could be utilized in future battles against this re-emerging pathogen which continues to cause devastating disease.
Related collections
Most cited references195
- Record: found
- Abstract: found
- Article: not found
Systems biology approach predicts immunogenicity of the yellow fever vaccine in humans.
- Record: found
- Abstract: found
- Article: not found
Composition and Three-Dimensional Architecture of the Dengue Virus Replication and Assembly Sites
- Record: found
- Abstract: found
- Article: found