- Record: found
- Abstract: found
- Article: found
PTH/SDF-1α cotherapy induces CD90+CD34− stromal cells migration and promotes tissue regeneration in a rat periodontal defect model
Read this article at
Abstract
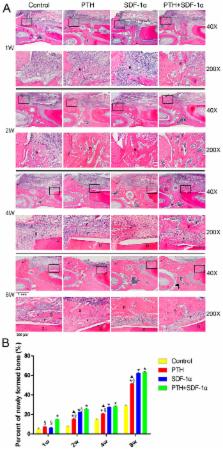
Stromal cell-derived factor-1α (SDF-1α) is a key stem cell homing factor that is crucial for recruitment of stem cells to many diseased organs. However, the therapeutic activity of SDF-1α is potentially limited by N-terminal cleavage at position-2 proline by a cell surface protein CD26/dipeptidyl peptidase-IV (DPP-IV). Parathyroid hormone (PTH) is a DPP-IV inhibitor and has been suggested as a promising agent for periodontal tissue repair. The purpose of this study was to explore the effects of a cell-free system comprising SDF-1α and scaffold plus PTH systemic application on periodontal tissue regeneration in vivo. The results showed that PTH/SDF-1α cotherapy improved the quantity of regenerated bone and resulted in better organization of ligament interface. We further investigated the possible mechanisms, and found that PTH/SDF-1α cotherapy enhanced CD90+CD34− stromal cells migration in vivo, increased the number of CXCR4 + cells in periodontal defects, induced early bone osteoclastogenesis and enhanced the expression of runt-related transcription factor 2 (Runx2), alkaline phosphatase (ALP) and collagen I (Col I) in newly formed bone tissue. In conclusion, this cell-free tissue engineering system with local administration of SDF-1α and systemic application of PTH could be employed to induce CD90+CD34− stromal cells recruitment and promote periodontal tissue regeneration.
Related collections
Most cited references34
- Record: found
- Abstract: found
- Article: not found
The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry.
- Record: found
- Abstract: found
- Article: not found
Regeneration of the articular surface of the rabbit synovial joint by cell homing: a proof of concept study.
- Record: found
- Abstract: found
- Article: not found