- Record: found
- Abstract: found
- Article: found
Regulation of the host immune system by helminth parasites
Read this article at
Abstract
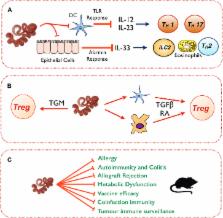
Helminth parasite infections are associated with a battery of immunomodulatory mechanisms that affect all facets of the host immune response to ensure their persistence within the host. This broad-spectrum modulation of host immunity has intended and unintended consequences, both advantageous and disadvantageous. Thus the host can benefit from suppression of collateral damage during parasite infection and from reduced allergic, autoimmune, and inflammatory reactions. However, helminth infection can also be detrimental in reducing vaccine responses, increasing susceptibility to coinfection and potentially reducing tumor immunosurveillance. In this review we will summarize the panoply of immunomodulatory mechanisms used by helminths, their potential utility in human disease, and prospective areas of future research.
Related collections
Most cited references139
- Record: found
- Abstract: found
- Article: not found
Tuft-cell-derived IL-25 regulates an intestinal ILC2-epithelial response circuit.
- Record: found
- Abstract: found
- Article: not found
Tuft cells, taste-chemosensory cells, orchestrate parasite type 2 immunity in the gut.
- Record: found
- Abstract: found
- Article: not found