- Record: found
- Abstract: found
- Article: found
PI3K/AKT/mTOR Signaling Pathway in Breast Cancer: From Molecular Landscape to Clinical Aspects
Read this article at
Abstract
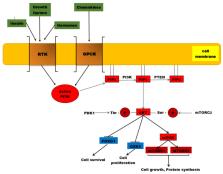
Breast cancer is a serious health problem worldwide, representing the second cause of death through malignancies among women in developed countries. Population, endogenous and exogenous hormones, and physiological, genetic and breast-related factors are involved in breast cancer pathogenesis. The phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT)/mammalian target of rapamycin (mTOR) is a signaling pathway involved in cell proliferation, survival, invasion, migration, apoptosis, glucose metabolism and DNA repair. In breast tumors, PIK3CA somatic mutations have been reported, located in exon 9 and exon 20. Up to 40% of PIK3CA mutations are estrogen receptor (ER) positive and human epidermal growth factor receptor 2 (HER2) -negative in primary and metastatic breast cancer. HER2 is overexpressed in 20–30% of breast cancers. HER1, HER2, HER3 and HER4 are membrane receptor tyrosine kinases involved in HER signaling to which various ligands can be attached, leading to PI3K/AKT activation. Currently, clinical studies evaluate inhibitors of the PI3K/AKT/mTOR axis. The main purpose of this review is to present general aspects of breast cancer, the components of the AKT signaling pathway, the factors that activate this protein kinase B, PI3K/AKT-breast cancer mutations, PI3K/AKT/mTOR-inhibitors, and the relationship between everolimus, temsirolimus and endocrine therapy.
Related collections
Most cited references203
- Record: found
- Abstract: found
- Article: not found
Molecular portraits of human breast tumours.
- Record: found
- Abstract: found
- Article: not found
Breast cancer statistics, 2019
- Record: found
- Abstract: found
- Article: not found