- Record: found
- Abstract: found
- Article: found
Increased oxidative stress mediates the antitumor effect of PARP inhibition in ovarian cancer

Read this article at
Abstract
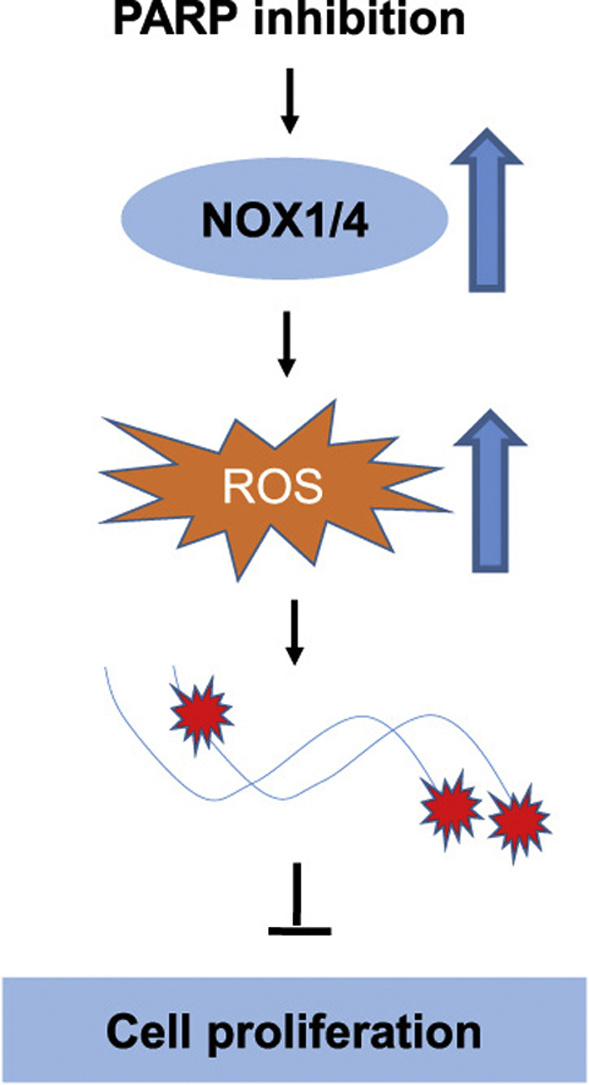
PARP inhibitors have been widely tested in clinical trials, especially for the treatment of breast cancer and ovarian cancer, and were shown to be highly successful. Because PARP primarily functions in sensing and repairing DNA strand breaks, the therapeutic effect of PARP inhibition is generally believed to be attributed to impaired DNA repair. We here report that oxidative stress is also increased by PARP inhibition and mediates the antitumor effect. We showed that PARP1 is highly expressed in specimens of high grade serous ovarian carcinoma and its activity is required for unperturbed proliferation of ovarian cancer cells. Inhibition or depletion of PARP leads to not only an increase in DNA damage, but also an elevation in the levels of reactive oxygen species (ROS). Importantly, antioxidant N-acetylcysteine (NAC) significantly attenuated the induction of DNA damage and the perturbation of proliferation by PARP inhibition or depletion. We further showed that NADPH oxidases 1 and 4 were significantly upregulated by PARP inhibition and were partially responsible for the induction of oxidative stress. Depletion of NOX1 and NOX4 partially rescued the growth inhibition of PARP1-deficient tumor xenografts. Our findings suggest that in addition to compromising the repair of DNA damage, PARP inhibition or depletion may exert extra antitumor effect by elevating oxidative stress in ovarian cancer cells.
Graphical abstract
Highlights
Related collections
Most cited references21
- Record: found
- Abstract: found
- Article: not found
Deficiency in the repair of DNA damage by homologous recombination and sensitivity to poly(ADP-ribose) polymerase inhibition.
- Record: found
- Abstract: found
- Article: not found
Defective mitophagy in XPA via PARP-1 hyperactivation and NAD(+)/SIRT1 reduction.
- Record: found
- Abstract: found
- Article: not found