- Record: found
- Abstract: found
- Article: found
KSR1 Knockout Mouse Model Demonstrates MAPK Pathway’s Key Role in Cisplatin- and Noise-induced Hearing Loss

Read this article at
Abstract
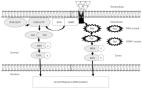
Hearing loss is a major disability in everyday life and therapeutic interventions to protect hearing would benefit a large portion of the world population. Here we found that mice devoid of the protein kinase suppressor of RAS 1 (KSR1) in their tissues (germline KO mice) exhibit resistance to both cisplatin- and noise-induced permanent hearing loss compared with their wild-type KSR1 littermates. KSR1 is a scaffold protein that brings in proximity the mitogen-activated protein kinase (MAPK) proteins BRAF, MEK1/2 and ERK1/2 and assists in their activation through a phosphorylation cascade induced by both cisplatin and noise insults in the cochlear cells. KSR1, BRAF, MEK1/2, and ERK1/2 are all ubiquitously expressed in the cochlea. Deleting the KSR1 protein tempered down the MAPK phosphorylation cascade in the cochlear cells following both cisplatin and noise insults and conferred hearing protection of up to 30 dB SPL in three tested frequencies in male and female mice. Treatment with dabrafenib, an FDA-approved oral BRAF inhibitor, protected male and female KSR1 wild-type mice from both cisplatin- and noise-induced hearing loss. Dabrafenib treatment did not enhance the protection of KO KSR1 mice, providing evidence dabrafenib works primarily through the MAPK pathway. Thus, either elimination of the KSR1 gene expression or drug inhibition of the MAPK cellular pathway in mice resulted in profound protection from both cisplatin- and noise-induced hearing loss. Inhibition of the MAPK pathway, a cellular pathway that responds to damage in the cochlear cells, can prove a valuable strategy to protect and treat hearing loss.
Related collections
Most cited references52
- Record: found
- Abstract: found
- Article: found
ERK/MAPK signalling pathway and tumorigenesis
- Record: found
- Abstract: not found
- Article: not found
ERK signalling: a master regulator of cell behaviour, life and fate

- Record: found
- Abstract: found
- Article: found