- Record: found
- Abstract: found
- Article: found
YTHDF3 facilitates triple-negative breast cancer progression and metastasis by stabilizing ZEB1 mRNA in an m 6A-dependent manner
Read this article at
Abstract
Background
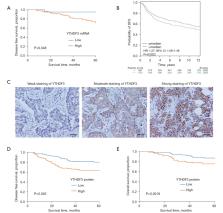
The YTH domain family protein 3 (YTHDF3) is an important N6-methyladenosine (m 6A) reader which is involved in multiple cancers. However, the biological role and mechanisms of action for YTHDF3 in triple-negative breast cancer (TNBC) remains to be elucidated.
Methods
The expression of YTHDF3 in TNBC tissues was evaluated using The Cancer Genome Atlas (TCGA) database, BC-GenExMiner, and immunohistochemistry (IHC) staining. Cell migration, invasion, and epithelial-mesenchymal transition (EMT) were validated by wound healing assays, transwell assays, and Western blot (WB) analyses. The association between YTHDF3 and zinc finger E-box-binding homeobox 1 (ZEB1) was confirmed by Pearson correlation analysis. RNA-binding protein immunoprecipitation (RIP) assays and mRNA actinomycin stability analyses were applied to confirm whether YTHDF3 could interact with ZEB1in an m 6A-dependent manner.
Results
The expression of YTHDF3 was correlated with poorer disease-free survival (DFS) and overall survival (OS) in TNBC patients. Functional experiments indicated that YTHDF3 positively regulated cell migration, invasion, and EMT in TNBC cells. Moreover, ZEB1 was identified as a key downstream target for YTHDF3 and YTHDF3 could enhance ZEB1 mRNA stability in an m 6A-dependent manner. Inhibition of YTHDF3 reduced migration, invasion, and EMT, all of which were reversed by rescue experiments overexpressing ZEB1.
Related collections
Most cited references45
- Record: found
- Abstract: found
- Article: not found
Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries
- Record: found
- Abstract: found
- Article: not found
m6A-dependent regulation of messenger RNA stability
- Record: found
- Abstract: found
- Article: not found
Dynamic RNA Modifications in Gene Expression Regulation
Author and article information
Comments
Comment on this article
 Smart Citations
Smart CitationsSee how this article has been cited at scite.ai
scite shows how a scientific paper has been cited by providing the context of the citation, a classification describing whether it supports, mentions, or contrasts the cited claim, and a label indicating in which section the citation was made.