- Record: found
- Abstract: found
- Article: found
Identification of a distinct desensitisation gate in the ATP-gated P2X2 receptor
Read this article at
Abstract
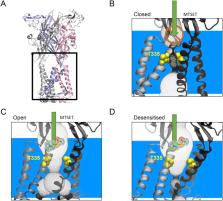
P2X receptors are trimeric ATP-gated ion channels. In response to ATP binding, conformational changes lead to opening of the channel and ion flow. Current flow can decline during continued ATP binding in a process called desensitisation. The rate and extent of desensitisation is affected by multiple factors, for instance the T18A mutation in P2X2 makes the ion channel fast desensitising. We have used this mutation to investigate whether the gate restricting ion flow is different in the desensitised and the closed state, by combining molecular modelling and cysteine modification using MTSET (2-(Trimethylammonium)ethyl methanethiosulfonate). Homology modelling of the P2X2 receptor and negative space imaging of the channel suggested a movement of the restriction gate with residue T335 being solvent accessible in the desensitised, but not the closed state. This was confirmed experimentally by probing the accessibility of T335C in the P2X2 T18A/T335C (fast desensitisation) and T335C (slow desensitisation) mutants with MTSET which demonstrates that the barrier to ion flow is different in the closed and the desensitised states. To investigate the T18A induced switch in desensitisation we compared molecular dynamics simulations of the wild type and T18A P2X2 receptor which suggest that the differences in time course of desensitisation are due to structural destabilization of a hydrogen bond network of conserved residues in the proximity of T18.
Graphical abstract
Highlights
Related collections
Most cited references29
- Record: found
- Abstract: found
- Article: not found
Crystal structure of the ATP-gated P2X4 ion channel in the closed state
- Record: found
- Abstract: found
- Article: not found
Molecular mechanism of ATP binding and ion channel activation in P2X receptors.
- Record: found
- Abstract: found
- Article: not found
