- Record: found
- Abstract: found
- Article: found
Potential‐Cycling Synthesis of Single Platinum Atoms for Efficient Hydrogen Evolution in Neutral Media
Read this article at
Abstract
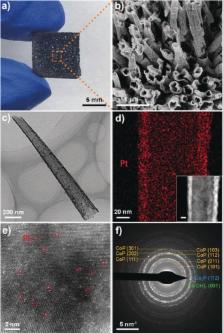
Single‐atom catalysts (SACs) have exhibited high activities for the hydrogen evolution reaction (HER) electrocatalysis in acidic or alkaline media, when they are used with binders on cathodes. However, to date, no SACs have been reported for the HER electrocatalysis in neutral media. We demonstrate a potential‐cycling method to synthesize a catalyst comprising single Pt atoms on CoP‐based nanotube arrays supported by a Ni foam, termed PtSA‐NT‐NF. This binder‐free catalyst is centimeter‐scale and scalable. It is directly used as HER cathodes, whose performances at low and high current densities in phosphate buffer solutions (pH 7.2) are comparable to and better than, respectively, those of commercial Pt/C. The Pt mass activity of PtSA‐NT‐NF is 4 times of that of Pt/C, and its electrocatalytic stability is also better than that of Pt/C. This work provides a large‐scale production strategy for binder‐free Pt SAC electrodes for efficient HER in neutral media.
Related collections
Most cited references37
- Record: found
- Abstract: found
- Article: not found
Thermally stable single-atom platinum-on-ceria catalysts via atom trapping
- Record: found
- Abstract: found
- Article: not found
Molecule-Level g-C3N4 Coordinated Transition Metals as a New Class of Electrocatalysts for Oxygen Electrode Reactions.
- Record: found
- Abstract: found
- Article: not found