- Record: found
- Abstract: found
- Article: found
Next-Generation Green Hydrogen: Progress and Perspective from Electricity, Catalyst to Electrolyte in Electrocatalytic Water Splitting
Read this article at
Highlights
-
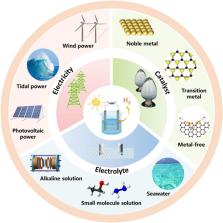
This review systematically summarizes the source of electricity, the key choice of catalyst, and the potentiality of electrolyte for prospective hydrogen generation.
-
Each section provides comprehensive overview, detailed comparison and obvious advantages in these system configurations.
-
The problems of hydrogen generation from electrolytic water splitting and directions of next-generation green hydrogen in the future are discussed and outlooked.
Abstract
Green hydrogen from electrolysis of water has attracted widespread attention as a renewable power source. Among several hydrogen production methods, it has become the most promising technology. However, there is no large-scale renewable hydrogen production system currently that can compete with conventional fossil fuel hydrogen production. Renewable energy electrocatalytic water splitting is an ideal production technology with environmental cleanliness protection and good hydrogen purity, which meet the requirements of future development. This review summarizes and introduces the current status of hydrogen production by water splitting from three aspects: electricity, catalyst and electrolyte. In particular, the present situation and the latest progress of the key sources of power, catalytic materials and electrolyzers for electrocatalytic water splitting are introduced. Finally, the problems of hydrogen generation from electrolytic water splitting and directions of next-generation green hydrogen in the future are discussed and outlooked. It is expected that this review will have an important impact on the field of hydrogen production from water.
Related collections
Most cited references358
- Record: found
- Abstract: found
- Article: not found
Enhancing hydrogen evolution activity in water splitting by tailoring Li⁺-Ni(OH)₂-Pt interfaces.
- Record: found
- Abstract: not found
- Article: not found
Emerging Two-Dimensional Nanomaterials for Electrocatalysis
- Record: found
- Abstract: found
- Article: not found
