- Record: found
- Abstract: found
- Article: found
lncRNA STEAP3-AS1 Modulates Cell Cycle Progression via Affecting CDKN1C Expression through STEAP3 in Colon Cancer
Read this article at
Abstract
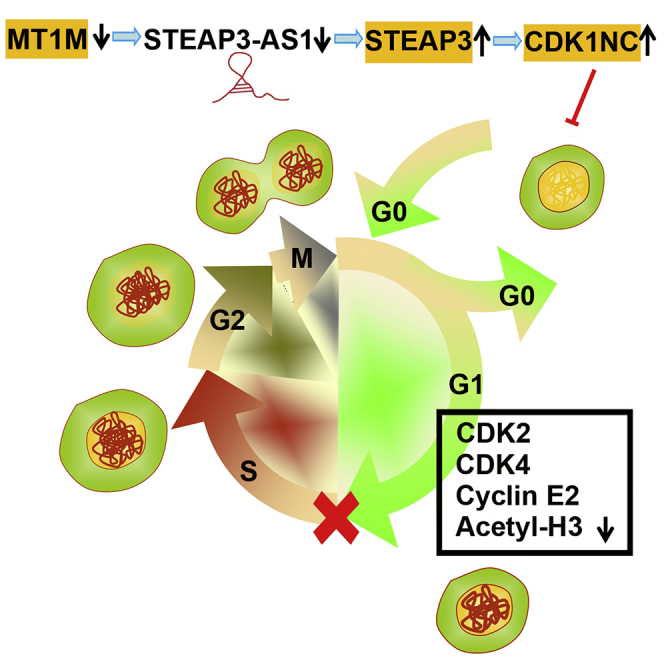
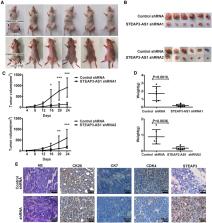
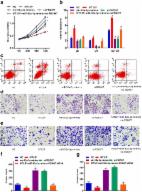
Previous studies have reported that long noncoding RNAs (lncRNAs) have acted as new players during tumorigenesis. Metallothionein also plays an important role in tumor progression. It is mainly considered to be involved in the process of cell proliferation, oxidative stress, and multidrug resistance. However, the potential involvement of metallothionein-related lncRNAs in colon cancer remains poorly understood. In our study, we found that MT1M affected the expression of lncRNA STEAP3-AS1. STEAP3-AS1 is located in physical contiguity with STEAP3 and notably increased in colon cancer tissues and cell lines. STEAP3-AS1 expression was negatively associated with the expression of STEAP3. High levels of STEPA3-AS1 were associated with poor overall survival in colon cancer patients. In in vitro assays, STEAP3-AS1 knockdown could inhibit colon cancer cell proliferation and migration and arrest colon cancer cells at the G 0–G 1 phase. In tumorigenicity assays, STEAP3-AS1 knockdown could strongly inhibit tumor growth. Mechanistic investigations demonstrated that STEAP3-AS1 downregulation could increase the expression of cyclin-dependent kinase inhibitor 1C (CDKN1C) by STEAP3 upregulation. Overall, we identify the underlying role of MT1M-related lncRNA STEAP3-AS1 in colon cancer progression, which provides a novel strategy for colon cancer therapy.
Graphical Abstract
Abstract
This work provides new findings about the function of lncRNA STEAP3-AS1 in colon cancer. Of relevance, overexpression STEPA3-AS1 was associated with poor overall survival in patients. The authors found that STEAP3-AS1 knockdown inhibited cell proliferation, migration, the cell cycle, and tumor growth. Mechanistically, STEAP3-AS1 downregulation increased CDKN1C expression by STEAP3 upregulation.
Related collections
Most cited references36
- Record: found
- Abstract: found
- Article: not found
CDK inhibitors: cell cycle regulators and beyond.
- Record: found
- Abstract: found
- Article: not found
Widespread occurrence of antisense transcription in the human genome.
- Record: found
- Abstract: found
- Article: found