- Record: found
- Abstract: found
- Article: found
Retinal vasoproliferative tumor regression after intravitreal aflibercept
Read this article at
Abstract
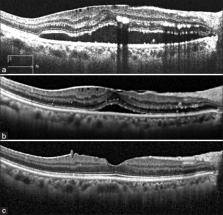
Retinal vasoproliferative tumors (RVPTs) are rare benign retinal lesions typically located in the inferotemporal peripheral retina. Several treatment options exist for the management of RVPTs, but no consensus has been proposed. There are only a few reports on the use of anti-vascular endothelial growth factor with bevacizumab to treat exudative or neovascular retinal changes secondary to RVPTs. This report describes a 68-year-old female with a history of systemic hypertension that presented with a 2-week history of gradual loss of visual acuity in the right eye. Fundoscopic examination showed a RVPTs with atypical location that had a favorable response to two-intravitreal aflibercept injections 1 month apart, with resulting subretinal fluid absorption and tumor regression.
Related collections
Most cited references17
- Record: found
- Abstract: found
- Article: not found
Humanization of an anti-vascular endothelial growth factor monoclonal antibody for the therapy of solid tumors and other disorders.
- Record: found
- Abstract: found
- Article: not found
VEGF-Trap: a VEGF blocker with potent antitumor effects.
- Record: found
- Abstract: found
- Article: not found