- Record: found
- Abstract: found
- Article: found
An inhibitory gate for state transition in cortex
Read this article at
Abstract
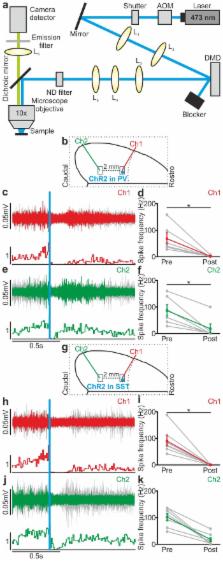
Large scale transitions between active (up) and silent (down) states during quiet wakefulness or NREM sleep regulate fundamental cortical functions and are known to involve both excitatory and inhibitory cells. However, if and how inhibition regulates these activity transitions is unclear. Using fluorescence-targeted electrophysiological recording and cell-specific optogenetic manipulation in both anesthetized and non-anesthetized mice, we found that two major classes of interneurons, the parvalbumin and the somatostatin positive cells, tightly control both up-to-down and down-to-up state transitions. Inhibitory regulation of state transition was observed under both natural and optogenetically-evoked conditions. Moreover, perturbative optogenetic experiments revealed that the inhibitory control of state transition was interneuron-type specific. Finally, local manipulation of small ensembles of interneurons affected cortical populations millimetres away from the modulated region. Together, these results demonstrate that inhibition potently gates transitions between cortical activity states, and reveal the cellular mechanisms by which local inhibitory microcircuits regulate state transitions at the mesoscale.
Related collections
Most cited references60
- Record: found
- Abstract: found
- Article: not found
High-Performance Genetically Targetable Optical Neural Silencing via Light-Driven Proton Pumps
- Record: found
- Abstract: found
- Article: not found
Prefrontal phase locking to hippocampal theta oscillations.
- Record: found
- Abstract: found
- Article: not found