- Record: found
- Abstract: found
- Article: found
Advances in immunology and immunotherapy for mesenchymal gastrointestinal cancers
Read this article at
Abstract
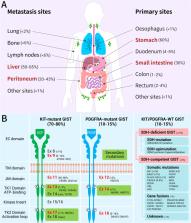
Mesenchymal gastrointestinal cancers are represented by the gastrointestinal stromal tumors (GISTs) which occur throughout the whole gastrointestinal tract, and affect human health and economy globally. Curative surgical resections and tyrosine kinase inhibitors (TKIs) are the main managements for localized GISTs and recurrent/metastatic GISTs, respectively. Despite multi-lines of TKIs treatments prolonged the survival time of recurrent/metastatic GISTs by delaying the relapse and metastasis of the tumor, drug resistance developed quickly and inevitably, and became the huge obstacle for stopping disease progression. Immunotherapy, which is typically represented by immune checkpoint inhibitors (ICIs), has achieved great success in several solid tumors by reactivating the host immune system, and been proposed as an alternative choice for GIST treatment. Substantial efforts have been devoted to the research of immunology and immunotherapy for GIST, and great achievements have been made. Generally, the intratumoral immune cell level and the immune-related gene expressions are influenced by metastasis status, anatomical locations, driver gene mutations of the tumor, and modulated by imatinib therapy. Systemic inflammatory biomarkers are regarded as prognostic indicators of GIST and closely associated with its clinicopathological features. The efficacy of immunotherapy strategies for GIST has been widely explored in pre-clinical cell and mouse models and clinical experiments in human, and some patients did benefit from ICIs. This review comprehensively summarizes the up-to-date advancements of immunology, immunotherapy and research models for GIST, and provides new insights and perspectives for future studies.
Related collections
Most cited references281
- Record: found
- Abstract: found
- Article: not found
Hallmarks of Cancer: New Dimensions
- Record: found
- Abstract: found
- Article: not found
PD-1 blockade induces responses by inhibiting adaptive immune resistance
- Record: found
- Abstract: found
- Article: not found