- Record: found
- Abstract: found
- Article: found
Structural and electronic trends of optical cycling centers in polyatomic molecules revealed by microwave spectroscopy of MgCCH, CaCCH, and SrCCH
Read this article at
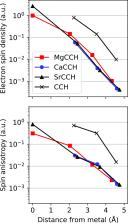
Significance
Laser-cooled molecules have enabled ground-breaking advances in quantum science. Transferring sophisticated optical cycling–based cooling techniques developed for simple diatomic molecules to complex polyatomic molecules remains a challenge, but will enable experiments of greater sensitivity and versatility. Achieving this goal requires a comprehensive understanding of the chemical properties that influence optical cycling efficiency. Using microwave rotational spectroscopy, we have derived precise structures and electronic properties for a family of metal–ligand radicals. We identify subtle but significant changes in bond lengths, electron distribution, and orbital hybridization to be quantitative measures of the ionic metal–ligand bonding character critical to efficient optical cycling. This work provides a foundation for elucidating transferable design principles for assessing how chemical modifications affect laser-cooling performance in larger molecules.
Abstract
The unique optical cycling efficiency of alkaline earth metal–ligand molecules has enabled significant advances in polyatomic laser cooling and trapping. Rotational spectroscopy is an ideal tool for probing the molecular properties that underpin optical cycling, thereby elucidating the design principles for expanding the chemical diversity and scope of these platforms for quantum science. We present a comprehensive study of the structure and electronic properties in alkaline earth metal acetylides with high-resolution microwave spectra of 17 isotopologues of MgCCH, CaCCH, and SrCCH in their 2 Σ + ground electronic states. The precise semiexperimental equilibrium geometry of each species has been derived by correcting the measured rotational constants for electronic and zero-point vibrational contributions calculated with high-level quantum chemistry methods. The well-resolved hyperfine structure associated with the 1,2H, 13C, and metal nuclear spins provides further information on the distribution and hybridization of the metal-centered, optically active unpaired electron. Together, these measurements allow us to correlate trends in chemical bonding and structure with the electronic properties that promote efficient optical cycling essential to next-generation experiments in precision measurement and quantum control of complex polyatomic molecules.
Related collections
Most cited references115
- Record: found
- Abstract: not found
- Article: not found
Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen
- Record: found
- Abstract: not found
- Article: not found
The fitting and prediction of vibration-rotation spectra with spin interactions
- Record: found
- Abstract: not found
- Article: not found