- Record: found
- Abstract: found
- Article: not found
Preparation of Fe 3O 4@SiO 2@ P(AANa- co-AM) Composites and Their Adsorption for Pb(II)

Abstract
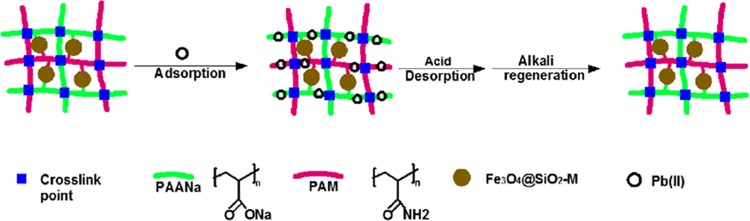
A series of magnetic composites of sodium polyacrylate and polyacrylamide copolymer [Fe 3O 4@SiO 2@P(AANa- co-AM)] were prepared. The investigation showed that the adsorption efficiency of Pb(II) was the best when the acrylamide/acrylic acid (AM/AA) mass ratio of composites was 5:5. Therefore, the composite of this ratio was selected as the adsorbent to systematically adsorb Pb(II) in aqueous solution. Static adsorption of Pb(II) to the magnetic composites in aqueous solutions was investigated by varying the solution pH and the concentration of Pb(II). The adsorption kinetics and isotherms model of Pb(II) on the Fe 3O 4@SiO 2@P(AANa- co-AM) composites followed a pseudo-second-order model and the Langmuir isotherm model, respectively. When the temperatures were 298.15, 308.15, and 318.15 K, the maximum adsorption capacities of Fe 3O 4@SiO 2@P(AANa- co-AM) composites were 237.53, 248.14, and 255.10 mg/g, respectively. The thermodynamic study of adsorption showed that the adsorption of Pb(II) on Fe 3O 4@SiO 2@P(AANa- co-AM) composites was a spontaneous endothermic process. The X-ray photoelectron spectroscopy (XPS) analysis showed that the adsorption of Pb(II) was due to the chelation between −COO – and Pb(II). After four adsorption–desorption cycles, the adsorbent can still maintain a high adsorption capacity.
Related collections
Most cited references35
- Record: found
- Abstract: not found
- Article: not found
Recent progress in sodium alginate based sustainable hydrogels for environmental applications
- Record: found
- Abstract: not found
- Article: not found
Magnetic EDTA-modified chitosan/SiO2/Fe3O4 adsorbent: Preparation, characterization, and application in heavy metal adsorption
- Record: found
- Abstract: found
- Article: not found
Current status of urban wastewater treatment plants in China.
Author and article information
Comments
Comment on this article
 Smart Citations
Smart CitationsSee how this article has been cited at scite.ai
scite shows how a scientific paper has been cited by providing the context of the citation, a classification describing whether it supports, mentions, or contrasts the cited claim, and a label indicating in which section the citation was made.