- Record: found
- Abstract: found
- Article: found
Selective neurodegeneration generated by intravenous α‐synuclein pre‐formed fibril administration is not associated with endogenous α‐synuclein levels in the rat brain

Read this article at
Abstract
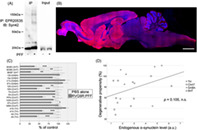
Selective loss of discrete neuronal populations is a prominent feature of many neurodegenerative conditions, but the molecular basis of this is poorly understood. A central role of α‐synuclein in the selective neurodegeneration of Parkinson's disease has been speculated, as its level of expression critically determines the propensity of this protein to misfold. To investigate whether the propensity of neuronal cell loss is associated with the level of endogenous α‐synuclein expression, non‐transgenic rats were given a single intravenous administration of α‐synuclein pre‐formed fibrils (PFFs) reversibly complexed with the rabies virus glycoprotein peptide (RVG9R). The number of surviving cells in different neuronal populations was systematically quantified using unbiased stereology. Our data demonstrated that a non‐selective, transvascular delivery of α‐synuclein PFFs led to a time‐dependent loss of specific populations of midbrain (but not olfactory) dopaminergic neurons, medullary (but not pontine) cholinergic neurons, and brainstem serotonergic neurons. Contrary to the central role of endogenous α‐synuclein expression in determining the seeding and aggregation propensity of pathological α‐synuclein, we did not observe an association between the levels of α‐synuclein expression in different regions of the rodent brain (although did not ascertain this at the individual cell level) and neurodegenerative propensity. The results from our study highlight the complexity of the neurodegenerative process generated by α‐synuclein seeding. Further investigations are therefore required to elucidate the molecular basis of neurodegeneration driven by exogenous pathogenic α‐synuclein spread.
Abstract
A non‐selective, transvascular delivery of α‐synuclein pre‐formed fibrils led to a time‐dependent loss of specific populations of neurons. However, we did not observe an association between the levels of α‐synuclein expression in different regions of the rodent brain and neurodegenerative propensity. Results from our study highlight the complexity of the neurodegenerative process generated by α‐synuclein seeding.
Related collections
Most cited references50
- Record: found
- Abstract: found
- Article: not found
Single-cell transcriptomic analysis of Alzheimer’s disease
- Record: found
- Abstract: found
- Article: not found
Pathological α-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice.
- Record: found
- Abstract: not found
- Article: not found