- Record: found
- Abstract: found
- Article: found
Peripheral oxytocin treatment ameliorates obesity by reducing food intake and visceral fat mass
Read this article at
Abstract
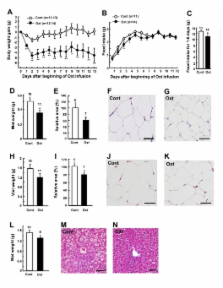
Recent studies suggest that oxytocin (Oxt) is implicated in energy metabolism. We aimed to explore acute and sub-chronic effects of peripheral Oxt treatment via different routes on food intake and energy balance. Intraperitoneal (ip) injection of Oxt concentration-dependently decreased food intake in mice. Ip Oxt injection induced c-Fos expression in the hypothalamus and brain stem including arcuate nucleus (ARC), paraventricular nucleus (PVN) and nucleus tractus solitarius (NTS). Subcutaneous (sc) injection of Oxt suppressed food intake in normal and high fat diet-induced obese (DIO) mice. Daily sc injection of Oxt for 17 days in DIO mice reduced food intake for 6 days and body weight for the entire treatment period and additional 9 days after terminating Oxt. Oxt infusion by sc implanted osmotic minipumps for 13 days in DIO mice reduced food intake, body weight, and visceral fat mass and adipocyte size. Oxt infusion also decreased respiratory quotient specifically in light phase, ameliorated fatty liver and glucose intolerance, without affecting normal blood pressure in DIO mice. These results demonstrate that peripheral Oxt treatment reduces food intake and visceral fat mass, and ameliorates obesity, fatty liver and glucose intolerance. Peripheral Oxt treatment provides a new therapeutic avenue for treating obesity and hyperphagia.
Related collections
Most cited references20
- Record: found
- Abstract: found
- Article: not found
Oxytocin, vasopressin, and the neurogenetics of sociality.
- Record: found
- Abstract: found
- Article: not found
Oxytocin receptor-deficient mice developed late-onset obesity.
- Record: found
- Abstract: found
- Article: not found