- Record: found
- Abstract: found
- Article: found
New developments and opportunities in drugs being trialed for amyotrophic lateral sclerosis from 2020 to 2022
Read this article at
Abstract
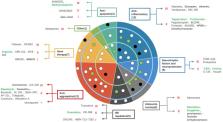
Amyotrophic lateral sclerosis (ALS) is a progressive neurodegenerative disorder that primarily affects motor neurons in the brain and spinal cord. In the recent past, there have been just two drugs approved for treatment, riluzole and edaravone, which only prolong survival by a few months. However, there are many novel experimental drugs in development. In this review, we summarize 53 new drugs that have been evaluated in clinical trials from 2020 to 2022, which we have classified into eight mechanistic groups (anti-apoptotic, anti-inflammatory, anti-excitotoxicity, regulated integrated stress response, neurotrophic factors and neuroprotection, anti-aggregation, gene therapy and other). Six were tested in phase 1 studies, 31 were in phase 2 studies, three failed in phase 3 studies and stopped further development, and the remaining 13 drugs were being tested in phase 3 studies, including methylcobalamin, masitinib, MN-166, verdiperstat, memantine, AMX0035, trazodone, CNM-Au8, pridopidine, SLS-005, IONN363, tofersen, and reldesemtiv. Among them, five drugs, including methylcobalamin, masitinib, AMX0035, CNM-Au8, and tofersen, have shown potent therapeutic effects in clinical trials. Recently, AMX0035 has been the third medicine approved by the FDA for the treatment of ALS after riluzole and edaravone.
Related collections
Most cited references171
- Record: found
- Abstract: not found
- Article: not found
Amyotrophic Lateral Sclerosis
- Record: found
- Abstract: found
- Article: not found
Necroptosis and RIPK1-mediated neuroinflammation in CNS diseases
- Record: found
- Abstract: not found
- Article: not found