- Record: found
- Abstract: found
- Article: found
Incidence and Estimated Vaccine Effectiveness Against Hospitalizations for All-Cause Pneumonia Among Older US Adults Who Were Vaccinated and Not Vaccinated With 13-Valent Pneumococcal Conjugate Vaccine
Read this article at
Abstract
This cohort study estimates the association between vaccination with 13-valent pneumococcal conjugate vaccine and hospitalizations for all-cause pneumonia and lower respiratory tract infection among older adults in a large health care system in California.
Key Points
Question
What is the estimated vaccine effectiveness of 13-valent pneumococcal conjugate vaccine (PCV13) against hospitalized all-cause pneumonia and lower respiratory tract infections (LRTI) in US adults aged 65 years or older?
Abstract
Importance
Following routine use of 13-valent pneumococcal conjugate vaccine (PCV13) in children in 2010, invasive pneumococcal disease rates have decreased substantially in children and adults. In 2014, the Advisory Committee for Immunization Practices recommended routine use of PCV13 among adults aged 65 years or older; previously only 23-valent pneumococcal polysaccharide vaccine (PPV23) was recommended.
Objective
To estimate the association between the incidence of hospitalized all-cause pneumonia and lower respiratory tract infections (LRTI) and PCV13 vaccination among older adults at Kaiser Permanente Northern California (KPNC).
Design, Setting, and Participants
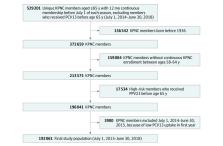
This retrospective cohort study included adults at KPNC aged 65 years or older between July 1, 2015, and June 30, 2018, born after 1936 with no known history of PPV23 or PCV13 receipt before age 65. The study took place at an integrated health care system with an annual membership more than 4 million individuals, approximately 15% of whom are 65 years or older and broadly representative of the region. Data analysis took place from July 2018 to December 2021, and data collection took place from November 2016 to June 2018.
Exposures
PCV13 vaccination status was ascertained from the electronic medical record (EMR). Individuals were considered vaccinated 14 days following immunization.
Main Outcomes and Measures
First hospitalized all-cause pneumonia was identified in the EMR using primary/secondary discharge diagnosis International Classification of Diseases, Ninth Revision and International Statistical Classification of Diseases and Related Health Problems, Tenth Revision codes. First hospitalized LRTI was identified using pneumonia codes and acute bronchitis codes. Relative risk (RR) of first pneumonia or LRTI hospitalization of individuals who were PCV13 vaccinated vs PCV13 unvaccinated was estimated using Poisson regressions adjusted for sex, race, ethnicity, age, influenza vaccine receipt, PPV23 receipt since age 65, pneumonia risk factors, health care use, and season. Vaccine effectiveness (VE) was estimated as (1–RR) × 100%.
Results
Of 192 061 adults, 107 957 (56%) were female and 139 024 (72%) were White individuals. PCV13 coverage increased from 0 in 2014 to 135 608 (76.9%) by 2018. There were 3488 individuals with 3766 pneumonia hospitalizations and 3846 individuals with 4173 LRTI hospitalizations. PCV13 was associated with an adjusted VE of 10.0% (95% CI, 2.4-17.0; P = .01) against hospitalized pneumonia and 9.4% (95% CI, 2.1-16.1; P = .01) against hospitalized LRTI.
Conclusions and Relevance
In the context of a robust pediatric PCV13 immunization program, PCV13 vaccination of adults aged 65 years or older was associated with significant reductions in hospitalizations for all-cause pneumonia and LRTI. Vaccinating older adults with PCVs may provide broader public health benefit against pneumonia hospitalizations.
Related collections
Most cited references43
- Record: found
- Abstract: found
- Article: not found
Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases
- Record: found
- Abstract: found
- Article: not found
Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults.
- Record: found
- Abstract: found
- Article: not found