- Record: found
- Abstract: found
- Article: found
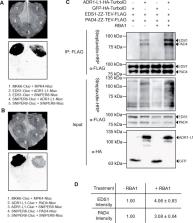
TIR signal promotes interactions between lipase-like proteins and ADR1-L1 receptor and ADR1-L1 oligomerization
letter
02 July 2021
Read this article at
There is no author summary for this article yet. Authors can add summaries to their articles on ScienceOpen to make them more accessible to a non-specialist audience.
Abstract
TIR signaling promotes the interactions between lipase-like proteins EDS1/PAD4 and ADR1-L1 immune receptor, and oligomerization of ADR1-L1.
Related collections
Most cited references16
- Record: found
- Abstract: found
- Article: not found
Intracellular innate immune surveillance devices in plants and animals.
Jonathan Jones, Russell E Vance, Jeffery Dangl (2016)
- Record: found
- Abstract: not found
- Article: not found
The ZAR1 resistosome is a calcium-permeable channel triggering plant immune signaling
- Record: found
- Abstract: found
- Article: not found
NAD+ cleavage activity by animal and plant TIR domains in cell death pathways
Shane Horsefield, Hayden Burdett, Xiaoxiao Zhang … (2019)