- Record: found
- Abstract: found
- Article: found
Long noncoding RNA MSC‐AS1 promotes hepatocellular carcinoma oncogenesis via inducing the expression of phosphoglycerate kinase 1

Read this article at
Abstract
Background and Objectives
Increasing studies report that lncRNAs are dysregulated in hepatocellular carcinoma (HCC), which might function as significant diagnostic biomarkers of HCC. LncRNA MSC‐AS1 has been newly discovered in several cancers. However, its biological effect in HCC remains to be clearly elucidated. The aim of our work was to test MSC‐AS1 expression level and assess its function in HCC progression.
Methods
Expression levels of MSC‐AS1 and PGK1 in HCC were tested by qRT‐PCR in HCC cells including HUH‐7, BEL‐7404, SNU449, HepG2, QGY‐7701, and human normal liver cells (HL‐7702 cells). Association of MSC‐AS1 expression with various clinicopathological features and patients’ survival were analyzed by chi‐squared test and Kaplan‐Meier, respectively. The functions of MSC‐AS1 in HCC cells were investigated using EdU assay, colony formation, flow cytometry, would healing assay, and Transwell matrigel invasion assays. The correlation between MSC‐AS1 and PGK1 was confirmed using a RIP assay. Protein expression of PGK1 was evaluated using a western blot assay.
Results
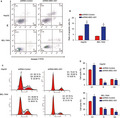
MSC‐AS1 was obviously upregulated in HCC tissues and HCC cells. Knockdown of MSC‐AS1 repressed HepG2 and BEL‐7404 cell proliferation, colony formation capacity, and triggered cell apoptosis. HepG2 and BEL‐7404 cell cycle was blocked in G1 phase and cell migration/invasion was remarkably depressed. Downregulation of MSC‐AS1 in HCC cells reduced PGK1 expression. In vivo data demonstrated that silence of MSC‐AS1 suppressed HCC development via activating PGK1.
Abstract
Related collections
Most cited references17
- Record: found
- Abstract: found
- Article: not found
LncRNA-Dependent Mechanisms of Androgen Receptor-regulated Gene Activation Programs
- Record: found
- Abstract: found
- Article: not found
The long noncoding RNA Malat1: Its physiological and pathophysiological functions.
- Record: found
- Abstract: found
- Article: not found