- Record: found
- Abstract: found
- Article: found
Preservation of myocardial contractile function by aminoguanidine, a nitric oxide synthase inhibitors, in a rat model of hemorrhagic shock
Read this article at
Abstract
Background: Myocardial contractile dysfunction plays a major role in the outcome of trauma patients. Nitric oxide (NO) has been shown to increase following haemorrhagic shock. Peroxynitrite which is produced by the reaction of NO with reactive oxygen species leads to nitrosative stress mediated organ injury and myocardial contractile dysfunction. Aminoguanidine is a selective inhibitor of inducible nitric oxide synthase (iNOS).
Objectives: The aim of this study was to determine the protective effects of inhibiting the production of NO using aminoguanidine (AG) on myocardial contractility, following hemorrhagic shock and resuscitation in rats.
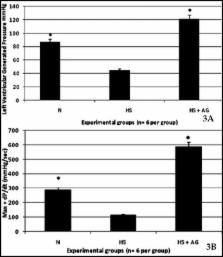
Methods: Male Sprague-Dawley rats were assigned to 3 experimental groups (n = 6 per group):1) Normotensive rats (N), 2) Hemorrhagic shock rats (HS), and 3) Hemorrhagic shock rats treated with AG 60 mg/kg AG intra-arterially (HS-AG). Rats were hemorrhaged over 60 minutes to reach a mean arterial blood pressure of 40 mmHg. Rats were treated with 1 ml of 60 mg/Kg AG intra-arterially after 60 minutes haemorrhagic shock,. Resuscitation was performed in vivo by the reinfusion of the shed blood for 30 minutes to restore normo-tension. Hearts were harvested and ex vivo perfused in the Langendorff System and myocardial function was determined by measuring the left ventricular end diastolic pressure (LVEDP) and left ventricular systolic pressure (LVSP). Left ventricular generated pressure (LVGP) and + dP/dt max was calculated.
Results: Hemorrhagic shock rats treated with AG exhibited a significant increase in left ventricular generated pressure LVGP (137.1 ± 9.4 mmHg) and + dP/dtmax (589.6 ± 110.7 mmHg/sec) compared with the untreated group (44.43 ± 20.18 mmHg, 289.8 ± 25.0 mmHg/sec).
Conclusion: Treatment with AG protects the myocardium from post-resuscitation myocardial dysfunction.
Related collections
Most cited references18
- Record: found
- Abstract: found
- Article: not found
Postinjury multiple organ failure: a bimodal phenomenon.
- Record: found
- Abstract: found
- Article: not found
Vascular hyporeactivity to vasoconstrictor agents and hemodynamic decompensation in hemorrhagic shock is mediated by nitric oxide.
- Record: found
- Abstract: found
- Article: not found