- Record: found
- Abstract: found
- Article: found
Mechanisms of Cardiovascular Benefits of Sodium Glucose Co-Transporter 2 (SGLT2) Inhibitors : A State-of-the-Art Review

Read this article at
Highlights
-
•
Treatment with SGLT2 inhibitors reduces the incidence of cardiovascular death and heart failure hospitalization in patients with and without diabetes.
-
•
This review discusses the potential mechanisms by which SGLT2 inhibitors exert their beneficial effects, including beneficial effects on cardiac energy metabolism, reducing inflammation, improving kidney function, and increasing erythropoiesis.
-
•
Future studies are required to clarify how SGLT2 inhibitors exert their impressive cardiovascular effects, which will allow for a more specific targeting of heart failure therapy.
Summary
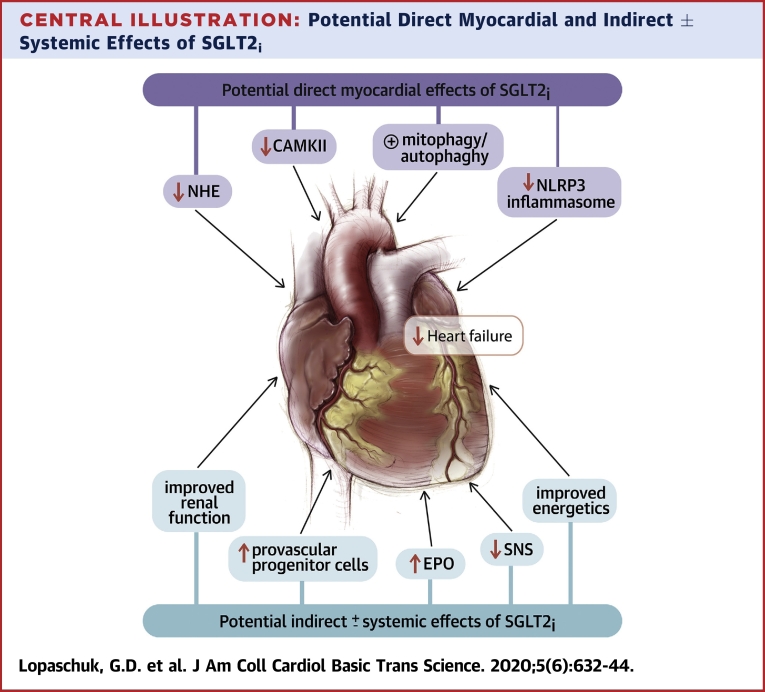
Recent clinical trials have shown that sodium glucose co-transport 2 (SGLT2) inhibitors have dramatic beneficial cardiovascular outcomes. These include a reduced incidence of cardiovascular death and heart failure hospitalization in people with and without diabetes, and those with and without prevalent heart failure. The actual mechanism(s) responsible for these beneficial effects are not completely clear. Several potential theses have been proposed to explain the cardioprotective effects of SGLT2 inhibition, which include diuresis/natriuresis, blood pressure reduction, erythropoiesis, improved cardiac energy metabolism, inflammation reduction, inhibition of the sympathetic nervous system, prevention of adverse cardiac remodeling, prevention of ischemia/reperfusion injury, inhibition of the Na +/H +-exchanger, inhibition of SGLT1, reduction in hyperuricemia, increasing autophagy and lysosomal degradation, decreasing epicardial fat mass, increasing erythropoietin levels, increasing circulating pro-vascular progenitor cells, decreasing oxidative stress, and improving vascular function. The strengths and weaknesses of these proposed mechanisms are reviewed in an effort to try to synthesize and prioritize the mechanisms as they relate to clinical event reduction.
Central Illustration
Related collections
Most cited references82
- Record: found
- Abstract: found
- Article: not found
Myocardial fatty acid metabolism in health and disease.
- Record: found
- Abstract: found
- Article: not found
SGLT2 inhibitors and mechanisms of cardiovascular benefit: a state-of-the-art review
- Record: found
- Abstract: found
- Article: not found