- Record: found
- Abstract: found
- Article: found
Alterations in adaptive immunity persist during long-duration spaceflight
Read this article at
Abstract
Background:
It is currently unknown whether immune system alterations persist during long-duration spaceflight. In this study various adaptive immune parameters were assessed in astronauts at three intervals during 6-month spaceflight on board the International Space Station (ISS).
AIMS:
To assess phenotypic and functional immune system alterations in astronauts participating in 6-month orbital spaceflight.
Methods:
Blood was collected before, during, and after flight from 23 astronauts participating in 6-month ISS expeditions. In-flight samples were returned to Earth within 48 h of collection for immediate analysis. Assays included peripheral leukocyte distribution, T-cell function, virus-specific immunity, and mitogen-stimulated cytokine production profiles.
Results:
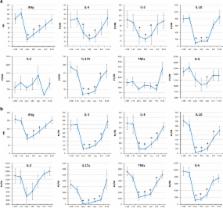
Redistribution of leukocyte subsets occurred during flight, including an elevated white blood cell (WBC) count and alterations in CD8 + T-cell maturation. A reduction in general T-cell function (both CD4 + and CD8 +) persisted for the duration of the 6-month spaceflights, with differential responses between mitogens suggesting an activation threshold shift. The percentage of CD4 + T cells capable of producing IL-2 was depressed after landing. Significant reductions in mitogen-stimulated production of IFNγ, IL-10, IL-5, TNFα, and IL-6 persisted during spaceflight. Following lipopolysaccharide (LPS) stimulation, production of IL-10 was reduced, whereas IL-8 production was increased during flight.
Related collections
Most cited references40
- Record: found
- Abstract: found
- Article: not found
Immune system dysregulation following short- vs long-duration spaceflight.
- Record: found
- Abstract: found
- Article: not found
Stress-induced subclinical reactivation of varicella zoster virus in astronauts.
- Record: found
- Abstract: found
- Article: not found