- Record: found
- Abstract: found
- Article: found
Repair of Damaged Articular Cartilage: Current Approaches and Future Directions
Read this article at
Abstract
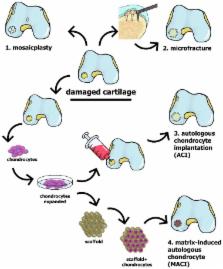
Articular hyaline cartilage is extensively hydrated, but it is neither innervated nor vascularized, and its low cell density allows only extremely limited self-renewal. Most clinical and research efforts currently focus on the restoration of cartilage damaged in connection with osteoarthritis or trauma. Here, we discuss current clinical approaches for repairing cartilage, as well as research approaches which are currently developing, and those under translation into clinical practice. We also describe potential future directions in this area, including tissue engineering based on scaffolding and/or stem cells as well as a combination of gene and cell therapy. Particular focus is placed on cell-based approaches and the potential of recently characterized chondro-progenitors; progress with induced pluripotent stem cells is also discussed. In this context, we also consider the ability of different types of stem cell to restore hyaline cartilage and the importance of mimicking the environment in vivo during cell expansion and differentiation into mature chondrocytes.
Related collections
Most cited references162
- Record: found
- Abstract: found
- Article: not found
Effect of Intra-articular Triamcinolone vs Saline on Knee Cartilage Volume and Pain in Patients With Knee Osteoarthritis: A Randomized Clinical Trial.
- Record: found
- Abstract: found
- Article: not found
Human gingiva-derived mesenchymal stem cells elicit polarization of m2 macrophages and enhance cutaneous wound healing.
- Record: found
- Abstract: found
- Article: not found