- Record: found
- Abstract: found
- Article: found
Exposome and unhealthy aging: environmental drivers from air pollution to occupational exposures
Read this article at
Abstract
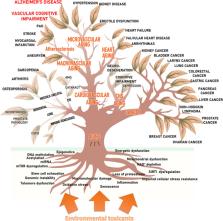
The aging population worldwide is facing a significant increase in age-related non-communicable diseases, including cardiovascular and brain pathologies. This comprehensive review paper delves into the impact of the exposome, which encompasses the totality of environmental exposures, on unhealthy aging. It explores how environmental factors contribute to the acceleration of aging processes, increase biological age, and facilitate the development and progression of a wide range of age-associated diseases. The impact of environmental factors on cognitive health and the development of chronic age-related diseases affecting the cardiovascular system and central nervous system is discussed, with a specific focus on Alzheimer’s disease, Parkinson’s disease, stroke, small vessel disease, and vascular cognitive impairment (VCI). Aging is a major risk factor for these diseases. Their pathogenesis involves cellular and molecular mechanisms of aging such as increased oxidative stress, impaired mitochondrial function, DNA damage, and inflammation and is influenced by environmental factors. Environmental toxicants, including ambient particulate matter, pesticides, heavy metals, and organic solvents, have been identified as significant contributors to cardiovascular and brain aging disorders. These toxicants can inflict both macro- and microvascular damage and many of them can also cross the blood–brain barrier, inducing neurotoxic effects, neuroinflammation, and neuronal dysfunction. In conclusion, environmental factors play a critical role in modulating cardiovascular and brain aging. A deeper understanding of how environmental toxicants exacerbate aging processes and contribute to the pathogenesis of neurodegenerative diseases, VCI, and dementia is crucial for the development of preventive strategies and interventions to promote cardiovascular, cerebrovascular, and brain health. By mitigating exposure to harmful environmental factors and promoting healthy aging, we can strive to reduce the burden of age-related cardiovascular and brain pathologies in the aging population.
Related collections
Most cited references361
- Record: found
- Abstract: found
- Article: found
The Hallmarks of Aging

- Record: found
- Abstract: found
- Article: found
Global burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019
- Record: found
- Abstract: found
- Article: not found