- Record: found
- Abstract: found
- Article: found
Optimized Surface Characteristics and Enhanced in Vivo Osseointegration of Alkali-Treated Titanium with Nanonetwork Structures
Read this article at
Abstract
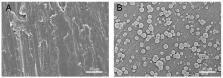
Alkali-treated titanium (Ti) with a porous, homogeneous, and uniform nanonetwork structure (TNS) that enables establishment of a more rapid and firmer osteointegration than titanium has recently been reported. However, the mechanisms underlying the enhanced osteogenic activity on TNS remains to be elucidated. This study aimed to evaluate the surface physicochemical properties of Ti and TNS, and investigate osteoinduction and osteointegration in vivo. Surface characteristics were evaluated using scanning electron microscopy (SEM), scanning probe microscopy (SPM), and X-ray photoelectron spectrometry (XPS), and the surface electrostatic force of TNS was determined using solid zeta potential. This study also evaluated the adsorption of bovine serum albumin (BSA) and human plasma fibronectin (HFN) on Ti and TNS surfaces using quartz crystal microbalance (QCM) sensors, and apatite formation on Ti and TNS surfaces was examined using a simulated body fluid (SBF) test. Compared with Ti, the newly developed TNS enhanced BSA and HFN absorbance capacity and promoted apatite formation. Furthermore, TNS held less negative charge than Ti. Notably, sequential fluorescence labeling and microcomputed tomography assessment indicated that TNS screws implanted into rat femurs exhibited remarkably enhanced osteointegration compared with Ti screws. These results indicate that alkali-treated titanium implant with a nanonetwork structure has considerable potential for future clinical applications in dentistry and orthopedics.
Related collections
Most cited references42
- Record: found
- Abstract: not found
- Article: not found
Ti based biomaterials, the ultimate choice for orthopaedic implants – A review
- Record: found
- Abstract: found
- Article: not found
Surface treatments of titanium dental implants for rapid osseointegration.
- Record: found
- Abstract: found
- Article: not found